Abstract
Purpose
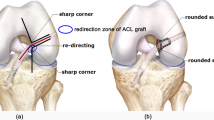
Tunnel enlargement and graft rupture are common complications associated with ACL reconstruction (ACLR). This study aims to explore how variations in graft stiffness and shape affect the strain energy density (SED) around bone tunnel entrances and stress on the graft and subsequently influencing the level of tunnel enlargement and graft wear.
Methods
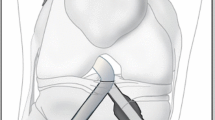
Finite element ACLR models were developed using different graft stiffnesses (323 N/mm, 545 N/mm and 776 N/mm) and shapes (circular and elliptical). The models were subjected to a combined loading of 103 N anterior tibial load, 7.5 Nm internal tibial moment, and 6.9 Nm valgus tibial moment at joint flexion of 30°. SED at tunnel entrances and stresses on the graft was recorded and compared among the different models.
Results
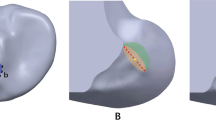
Increasing the graft stiffness resulted in greater stress on the graft (17.2, 24.4 and 31.7 MPa for graft stiffnesses of 323 N/mm, 545 N/mm and 776 N/mm), but had little effect on the SED reduction around the tunnel entrances. Changing the cross section of the graft from circular to elliptical caused an additional reduction in SED (56.8 vs 2.8 kJ/m3) at the posterior zone of the femoral tunnel entrance and increased the stress on the graft (31.7 MPa vs 38.9 MPa).
Conclusions
This study recommends using ACL grafts with lower stiffness and a circular cross section to reduce tunnel enlargement and graft wear following ACLR.






Similar content being viewed by others
References
Amis AA, Kempson SA (1999) Failure mechanisms of polyester fiber anterior cruciate ligament implants: A human retrieval and laboratory study. J Biomed Mater Res 48:534–539
Archard JF (1953) Contact and rubbing of flat surfaces. J Appl phys 24:981–988
Dericks G (1995) Ligament advanced reinforcementsystem anterior cruciate ligament reconstruction. Oper Tech Sports Med 3:187–205
Fink C, Zapp M, Benedetto KP, Hackl W, Hoser C, Rieger M (2001) Tibial tunnel enlargement following anterior cruciate ligament reconstruction with patellar tendon autograft. Arthroscopy 17:138–143
Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, DeMaio M, Dick RW, Engebretsen L, Garrett WE, Hannafin JA (2006) Understanding and preventing noncontact anterior cruciate ligament injuries a review of the Hunt Valley II meeting, January 2005. Am J Sports Med 34:1512–1532
Guidoin MF, Marois Y, Bejui J, Poddevin N, King MW, Guidoin R (2000) Analysis of retrieved polymer fiber based replacements for the ACL. Biomaterials 21:2461–2474
Hamner DL, Brown CH, Steiner ME, Hecker AT, Hayes WC (1999) Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am 81:549–557
Herzog MM, Marshall SW, Lund JL, Pate V, Mack CD, Spang JT (2017) Incidence of anterior cruciate ligament reconstruction among adolescent females in the United States, 2002 through 2014. JAMA Pediatr 171:808–810
Hoshino Y, Kuroda R, Nishizawa Y, Nakano N, Nagai K, Araki D, Oka S, Kawaguchi S, Nagamune K, Kurosaka M (2018) Stress distribution is deviated around the aperture of the femoral tunnel in the anatomic anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 26:1145–1151
Huiskes R, Weinans H, Van RB (1992) The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res 274:124–134
Jagodzinski M, Foerstemann T, Mall G, Krettek C, Bosch U, Paessler H (2005) Analysis of forces of ACL reconstructions at the tunnel entrance: is tunnel enlargement a biomechanical problem? J Biomech 38:23–31
Jakobsen T, Kold S, Shiguetomi-Medina J, Baas J, Soballe K, Rahbek O (2017) Topical zoledronic acid decreases micromotion induced bone resorption in a sheep arthroplasty model. BMC Musculoskel Dis 18:441–447
Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SLY (1994) Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 12:796–803
Kiapour AM, Kaul V, Kiapour A, Quatman CE, Wordeman SC, Hewett TE, Demetropoulos CK, Goel VK (2014) The effect of ligament modeling technique on knee joint kinematics: a finite element study. Appl Math 4:91–97
Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, Bergmann G (2010) Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech 43:2164–2173
Lai CC, Ardern CL, Feller JA, Webster KE (2018) Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med 52:128–138
Li G, Gil J, Kanamori A, Woo SLY (1999) A validated three-dimensional computational model of a human knee joint. J Biomech Eng 121:657–662
Lin CL, Lin YS (2010) Multi-factorial analysis of variables influencing the bone loss of an implant placed in the maxilla: prediction using FEA and SED bone remodeling algorithm. J Biomech 43:644–651
Muren O, Dahlstedt L, Brosjö E, Dahlborn M, Dalén N (2005) Gross osteolytic tibia tunnel widening with the use of Gore-Tex anterior cruciate ligament prosthesis: a radiological, arthrometric and clinical evaluation of 17 patients 13–15 years after surgery. Acta Orthop 76:270–274
Nebelung W, Becker R, Merkel M, RoPke M (1998) Bone tunnel enlargement after anterior cruciate ligament reconstruction with semitendinosus tendon using Endobutton fixation on the femoral side. Arthroscopy 14:810–815
Parchi PD, Ciapini G, Paglialunga C, Giuntoli M, Picece C, Chiellini F, Lisanti M, Scaglione M (2018) Anterior cruciate ligament reconstruction with LARS artificial ligament-clinical results after a long-term follow-up. Joints 6:75–79
Pioletti DP, Rakotomanana L, Benvenuti J, Leyvraz P (1998) Viscoelastic constitutive law in large deformations: application to human knee ligaments and tendons. J Biomech 31:753–757
Quapp KM, Weiss JA (1998) Material characterization of human medial collateral ligament. J Biomech Eng 120:757–763
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20:92–102
Sasaki N, Farraro KF, Kim KE, Woo SLY (2014) Biomechanical evaluation of the quadriceps tendon autograft for anterior cruciate ligament reconstruction: a cadaveric study. Am J Sports Med 42:723–730
Sauer S, Lind M (2017) Bone tunnel enlargement after ACL reconstruction with hamstring autograft is dependent on original bone tunnel diameter. Surg J 03:e96–e100
Song Y, Debski RE, Musahl V, Thomas M, Woo SLY (2004) A three-dimensional finite element model of the human anterior cruciate ligament: a computational analysis with experimental validation. J Biomech 37:383–390
Srinivas DK, Kanthila M, Saya RP, Vidyasagar J (2016) Femoral and tibial tunnel widening following anterior cruciate ligament reconstruction using various modalities of fixation: a prospective observational study. J Clin Diagn Res 10:RC09–RC11
Tachibana Y, Mae T, Shino K, Kanamoto T, Sugamoto K, Yoshikawa H, Nakata K (2014) Morphological changes in femoral tunnels after anatomic anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 23:3591–3600
Weber AE, Delos D, Oltean HN, Vadasdi K, Cavanaugh J, Potter HG, Rodeo SA (2015) Tibial and femoral tunnel changes after ACL reconstruction: a prospective 2-year longitudinal MRI study. Am J Sports Med 43:1147–1156
Woo SLY, Hollis JM, Adams DJ, Lyon RM, Takai S (1991) Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med 19:217–225
Yao J, Snibbe J, Maloney M, Lerner AL (2006) Stresses and strains in the medial meniscus of an ACL deficient knee under anterior loading: a finite element analysis with image-based experimental validation. J Biomech Eng 128:135–141
Yao J, Wen C, Cheung JT, Zhang M, Hu Y, Yan C, Chiu KY, Lu WW, Fan Y (2012) Deterioration of stress distribution due to tunnel creation in single-bundle and double-bundle anterior cruciate ligament reconstructions. Ann Biomed Eng 40:1554–1567
Acknowledgements
Cadaveric experiments for model validation were performed at MSRC, University of Pittsburgh. The authors would like to thank Dr. Savio L-Y. Woo for his kind support in sourcing samples and equipment. We would also like to thank Dr. Jonquil R. Mau and Dr. Huijun Kang for their help with robotic testing. Mr. Colin McClean is acknowledged for his assistance with editing this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
All procedures performed in this study involving human cadaveric knees were approved by the Committee for Oversight of Research and Clinical Training Involving Decedents in the University of Pittsburgh (CORID No. 222) and were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, H., Zhang, B. & Cheng, CK. Stiffness and shape of the ACL graft affects tunnel enlargement and graft wear. Knee Surg Sports Traumatol Arthrosc 28, 2184–2193 (2020). https://doi.org/10.1007/s00167-019-05772-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05772-0