Abstract
Purpose
Sepsis is recognized as a global public health problem, but the proportion due to hospital-acquired infections remains unclear. We aimed to summarize the epidemiological evidence related to the burden of hospital-acquired (HA) and ICU-acquired (ICU-A) sepsis.
Methods
We searched MEDLINE, Embase and the Global Index Medicus from 01/2000 to 03/2018. We included studies conducted hospital-wide or in intensive care units (ICUs), including neonatal units (NICUs), with data on the incidence/prevalence of HA and ICU-A sepsis and the proportion of community and hospital/ICU origin. We did random-effects meta-analyses to obtain pooled estimates; inter-study heterogeneity and risk of bias were assessed.
Results
Of the 13,239 studies identified, 51 met the inclusion criteria; 22 were from low- and middle-income countries. Twenty-eight studies were conducted in ICUs, 13 in NICUs, and ten hospital-wide. The proportion of HA sepsis among all hospital-treated sepsis cases was 23.6% (95% CI 17–31.8%, range 16–36.4%). In the ICU, 24.4% (95% CI 16.7–34.2%, range 10.3–42.5%) of cases of sepsis with organ dysfunction were acquired during ICU stay and 48.7% (95% CI 38.3–59.3%, range 18.7–69.4%) had a hospital origin. The pooled hospital incidence of HA sepsis with organ dysfunction per 1000 patients was 9.3 (95% CI 7.3–11.9, range 2–20.6)). In the ICU, the pooled incidence of HA sepsis with organ dysfunction per 1000 patients was 56.5 (95% CI 35–90.2, range 9.2–254.4) and it was particularly high in NICUs. Mortality of ICU patients with HA sepsis with organ dysfunction was 52.3% (95% CI 43.4–61.1%, range 30.1–64.6%). There was a significant inter-study heterogeneity. Risk of bias was low to moderate in ICU-based studies and moderate to high in hospital-wide and NICU studies.
Conclusion
HA sepsis is of major public health importance, and the burden is particularly high in ICUs. There is an urgent need to improve the implementation of global and local infection prevention and management strategies to reduce its high burden among hospitalized patients.
Similar content being viewed by others

In ICUs worldwide, hospital-acquired sepsis is a frequent adverse outcome with high mortality (exceeding 40%) and increased length of stay. There is urgent need to improve the implementation of global and local infection prevention and control strategies to reduce the burden of healthcare-associated infections, as well as approaches for their early diagnosis and adequate treatment to prevent a progression to sepsis complications. |
Introduction
Sepsis is defined as a life-threatening syndrome associated with physiological, pathological and biological abnormalities caused by a dysregulated host response to infections [1]. It is a global public health concern due to its high mortality and morbidity, and substantial economic burden [2]. Rudd and colleagues recently reported the shocking global estimates of 48.9 million cases of sepsis in 2017 and 11.0 million sepsis-related deaths [3]. According to a systematic review published in 2016 and based on studies from high-income countries, more than 30 million cases of hospital-treated sepsis are estimated to occur every year worldwide, with 5.3 million patients dying from sepsis [4].
Sepsis is also of great significance in the intensive care unit (ICU), where it affects approximately 30% of patients, with large variations between different geographical regions [5]. A study based in the USA with more than 170,000 sepsis cases reported that 55% of all sepsis cases required ICU admission [6]. Although it occurs across all age groups, the burden of sepsis is especially high among neonates [7].
Sepsis can occur as a complication of infections acquired in the community, which is reported to represent up to 70% of all sepsis cases according to Reinhart and colleagues [2]. It can also develop from healthcare-associated infections (HAIs) that are mostly preventable by appropriate infection prevention and control (IPC) measures [8]. According to a 2011 global report by the World Health Organization (WHO), HAIs prevalence varies between 5.7 and 19.1% hospital-wide [9]. More recent data show that in Europe [10] and the USA [11] hospital-wide prevalence of HAIs is 6.5% and 3.2%, respectively. A multicentre prospective study in ICUs in Brazil showed that 60% of sepsis cases were from HAIs, suggesting that HAIs relatively play a more significant role in epidemiological burden in low- and middle-income countries [12].
Importantly, recent data showed that up to 55% of all HAIs can be prevented by the implementation of multifaceted IPC interventions [13], which would ultimately result in a significant reduction in hospital-acquired sepsis (HA sepsis) cases. However, most sepsis studies lack the differentiation between community-acquired and HA sepsis [3, 4], and no systematic review on the global burden of HA sepsis has been conducted yet, including in the ICU setting.
Therefore, we conducted a systematic review and meta-analysis to assess the prevalence, incidence, patient length of stay and mortality of HA sepsis worldwide and to describe causative organisms, including antimicrobial resistance (AMR) patterns.
Methods
This systematic review followed a protocol published in the Prospective Register for Systematic Reviews (PROSPERO 2018 CRD42018089554) and was performed according to the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [14].
Search strategy
We systematically searched MEDLINE, EMBASE and the Global Index Medicus (African Index Medicus, Index Medicus for the Eastern Mediterranean Region, Index Medicus for the South-East Asia Region, Latin America and the Caribbean Literature on Health Sciences and Western Pacific Region Index Medicus) for studies published from 1 Jan 2000 to 7 March 2018 (date of last search). Language was restricted to Arabic, English, French, German, Italian, Japanese, Portuguese, Russian or Spanish. Details of the complete search strategy are presented in Supplementary material 1. Potentially relevant articles were retrieved for full-text review. Search results (titles, abstracts, full texts) were independently assessed by at least two investigators (RM, TH, HS, ST). Discordances were solved by a third reviewer or by discussion.
Study selection criteria
Studies, including full-text publications and conference abstracts, were included if they met all of the following criteria. (1) Data reported on the incidence or prevalence of HA sepsis (the condition had to be named “sepsis”, “severe sepsis” or “septic shock” or similar). (2) Sepsis in children and adults defined according to appropriate sepsis definitions (such as consensus definitions like sepsis-1 [15], -2 [16], -3 [1]) or identified with appropriate International Classification of Disease (ICD) codes (Supplementary material 2, Table 1) [17, 18]. Apart from the diagnosis of clinical sepsis in neonates, studies defining “clinical sepsis” according to the criteria for HAIs of the United States Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network (NHSN) [19] were excluded, as this definition only represents a subgroup of healthcare-associated primary bloodstream infections. Due to the difficulty regarding the definition of sepsis in neonates and the lack of validated consensus definitions, we included all studies on neonatal sepsis in neonatal ICUs (NICUs) if their sepsis case definition was based on clinical criteria of systemic infections (e.g. fever, hypothermia, bradycardia, apnoea, etc.). (3) The study could be of any design, apart from a randomised controlled trial, case series or case–control study, and had to provide original data. (4) Data collection had to be finished after 1 January 2000. (5) The study was conducted hospital-wide or in ICUs (including paediatric and NICUs) with largely unselected patient cohorts, i.e. not only high-risk populations (e.g. low birthweight neonates) or those with a specific underlying disease (e.g. cancer). (6) The study provided data at least related to the defined primary outcomes of this systematic review.
Definitions used in this study
For the purpose of this study, “hospital-acquired” is defined as a case of infection/sepsis acquired in the hospital, including ICUs, while “ICU-acquired” denotes a subset of hospital-acquired infections/sepsis and comprises all cases of infection/sepsis acquired during ICU stay. Any reported timescale of “hospital-acquired” was accepted. In the included studies, “hospital-acquired” and “ICU-acquired” were usually defined as disease onset occurring 48–72 h after hospital and ICU admission, respectively.
In this study, “sepsis” is an umbrella term for cases of sepsis, sepsis with organ dysfunction and septic shock. Similarly, “sepsis with organ dysfunction” is an umbrella term for cases of sepsis with organ dysfunction and septic shock. Importantly, cases of “severe sepsis” defined here by sepsis-1 and sepsis-2 definitions were termed “sepsis with organ dysfunction”. As the current sepsis-3 definition includes organ dysfunction as part of its sepsis case definition, “sepsis” cases in these studies were designated as “sepsis with organ dysfunction” cases.
Study outcomes
Primary outcomes were population and/or hospital-wide/ICU incidence, incidence density and/or prevalence of HA sepsis; proportion of HA sepsis (1) among all sepsis patients (both of community and hospital origin) or (2) among all patients with HAIs. Secondary outcomes were (1) attributable and crude mortality; (2) length of stay; (3) microbiological data, including data on AMR of microorganisms isolated from sepsis patients.
Data extraction and risk of bias assessment
From eligible studies, at least two independent reviewers (RM, TH, HS, ST) extracted data on the primary and secondary outcomes and the following study characteristics using standardized forms: study location (including WHO region and income level according to the World Bank [20]; study design; study period; patient inclusion and exclusion criteria; age group; sepsis case definition used; study sponsorship; conflict of interests; infection origin (i.e. hospital-acquired or ICU-acquired); and blood culture status (studies in NICUs). The risk of bias of individual studies was assessed using the tool developed by Hoy et al. [21]. After the initial PROSPERO registration, we modified the protocol and decided not to use the GRADE methodology to assess the quality of evidence because of methodological uncertainties in the application of GRADE to incidence and prevalence studies [22].
Statistical analysis
HA sepsis types were categorized into sepsis, sepsis with organ dysfunction and septic shock. Studies were grouped into hospital-wide, ICU-based and NICU-based. Pooled estimates were calculated using a random-effects model with logit-transformed raw proportions, and between-study variance τ2 was estimated using the DerSimonian–Laird estimator. Statistical heterogeneity was quantified using I2 statistics. All statistical analyses were performed using R (version 3.6.1) and the “meta” package (version 4.9.5).
Role of the funding source
WHO provided funding for the study and acted as a consultant in study design, data extraction, data interpretation and writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
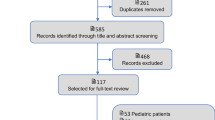
Of the 13,239 articles identified in our search, 1752 qualified for full-text review following title and abstract screening, of which 51 [6, 12, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] were included in the systematic review (Fig. 1). A summary of all outcomes studied, including the respective number of studies reporting data for each outcome, is provided in Supplementary material 2, Fig. 1.
Study characteristics
Among the included studies, 28 were conducted in ICUs (including adult or paediatric ICU patients) [12, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58], 13 in NICUs [59,60,61,62,63,64,65,66,67,68,69,70,71] and 10 were conducted hospital-wide (including patients from all hospital wards) [6, 23,24,25,26,27,28,29,30,31] (Supplementary material 2, Table 1). Most hospital-wide and ICU-based studies were conducted in high-income countries (n = 26/38) and nations from the European and American WHO regions (n = 29/38) (Fig. 2; Supplementary material 2, Table 1). The studies were carried out between 1997 and 2014. Only two studies were from the WHO Eastern Mediterranean region [33, 38], and only one from the South-East Asia region [23]; no eligible study was identified from the WHO Africa region. Thirty-two of 38 hospital-wide and ICU-based studies were multicentre trials, including one large international study [72] with data from 730 ICUs from 84 countries worldwide. Thirty-three of 38 hospital-wide and ICU-based studies relied on consensus sepsis definitions (22 × sepsis-1 [15], 3 × sepsis-2 [16], 2 × sepsis-3 [1], 4 × sepsis-1/-2 consensus definitions, and two used the 2005 definition of International Pediatric Sepsis Definition Consensus Conference [73]); of the remaining five, one study used a modified definition [50] and four hospital-wide studies used ICD-9-based case definitions(Supplementary material 2, Table 1). All ICU-based studies used clinical sepsis definitions.
Studies on HA neonatal sepsis in NICUs were distributed across all WHO regions, apart from the WHO Africa region (Fig. 2; Supplementary material Table 1), and were conducted between 1999 and 2013. By contrast to the adult and paediatric ICU studies, most (n = 10/13) neonatal sepsis reports were from low-income (n = 2) and middle-income (n = 8) countries and represented single-centre trials (n = 12/13). Neonatal sepsis case definitions varied. Eight studies used clinical criteria as defined by the CDC/NHSN [19], while the others used modified criteria.
Risk of bias assessment
In hospital-wide studies, the overall risk of bias was moderate to high (Supplementary material 2, Table 2) except for one study [6] that was judged as low risk of bias. National representativeness was unclear or low in most studies. In four of 10 hospital-wide studies, sepsis cases were identified using ICD codes. Although ICD codes are often used to identify sepsis cases, the accuracy for HA sepsis case identification remains unknown, and thus, risk of bias of the ICD-based case definitions was judged as high. The overall risk of bias of the ICU-based studies was low to moderate. Although the majority of ICU studies were multicentre trials, national representativeness was unclear or low in most reports. Since all ICU-based studies on paediatric and/or adult sepsis used case definitions based on clinical consensus definitions, risk of bias for the applied case definition was ranked as low. Except for one study [67], the overall risk of bias was high in all neonatal studies due to low national representativeness and the unknown accuracy of the applied neonatal sepsis case definitions. Results for all outcomes studied are summarized in Table 1 and Supplementary material 2 (Tables 3-6), including pooled estimates and I2 statistics. Results related to the studies [28,29,30, 32, 33, 38, 49, 52, 53, 58, 68] reporting data on the length of stay (LOS) of patients with HA sepsis as well as those including data on the microbiological profile, including antimicrobial resistance, of hospital-acquired sepsis or sepsis with organ dysfunction [23, 38, 48, 53, 55, 59, 69] are reported in the Supplementary material 3, Tables 1 and 2, respectively.
Hospital-wide and ICU incidence of HA sepsis per 1000 patients
We identified eight hospital-wide studies [6, 23,24,25,26,27, 29, 31] (including ICUs) that provided data on the incidence of HA sepsis or HA sepsis with organ dysfunction (Fig. 3a; Table 1). As reported by four studies [23, 24, 26, 31], the pooled incidence of HA sepsis was 15.4 (95% CI 9.2–25.7) cases per 1000 patients with individual study estimates ranging from 7.4 to 29.5 cases per 1000 patients. Based on five studies [6, 24, 25, 29, 74], the pooled hospital-wide incidence of HA sepsis with organ dysfunction was 9.3 (95% CI 7.3–11.9, range 2–20.6) cases per 1000 patients. One Spanish multicentre study [24] provided data on the incidence of HA septic shock and reported 1.0 cases per 1000 patients.
Pooled incidence of hospital-acquired sepsis per 1000 patients in different settings. a Pooled incidence of hospital-acquired sepsis, sepsis with organ dysfunction and septic shock among patients admitted to any ward in the hospital. b Pooled incidence of ICU-acquired and hospital-acquired sepsis, sepsis with organ dysfunction and septic shock among patients admitted to the ICU. The “sepsis” group comprises studies on patients with sepsis, sepsis with organ dysfunction and septic shock. The “sepsis with organ dysfunction” group comprises studies on patients with sepsis with organ dysfunction and septic shock. HA hospital-acquired, ICU-A ICU-acquired, 95% CI 95% confidence interval
In the ICU setting, nineteen studies [32,33,34,35, 37, 38, 40, 41, 43, 44, 47,48,49, 51,52,53,54, 57, 58, 72, 75] reported data on the incidence of ICU-acquired and/or HA sepsis (Fig. 3b and Table 1). The pooled incidence of ICU-acquired sepsis was 44.8 (95% CI 25.5–77.4) cases per 1000 ICU patients (seven studies [40, 43, 47, 49, 51, 53, 75]) with individual study estimates ranging from 8 to 90.4 cases per 1000 ICU patients. The pooled incidence of ICU-acquired sepsis with organ dysfunction determined from 12 studies [32,33,34,35, 37, 41, 47, 48, 52, 57, 58, 72] was 35.8 (95% CI 19.1–66.3, range 5.0–373.2) cases per 1000 ICU patients. Of note, based on data from 730 ICUs from 84 countries, Vincent and colleagues [72] showed a worldwide incidence of ICU-acquired sepsis with organ dysfunction of 62.3 cases per 1000 ICU patients. For ICU-acquired septic shock, the pooled estimate from two studies [47, 58] was 20.3 (95% CI 0.9–317.1) cases per 1000 ICU patients. Eleven studies [32,33,34,35, 38, 41, 44, 48, 52, 54, 57] provided data on ICU-treated HA sepsis with organ dysfunction (acquired in all hospital wards, including ICU) and found a pooled incidence of 56.5 (95% CI 35–90.2, range 9.2–254.4) cases per 1000 ICU patients. No statistically significant differences were found in pooled summaries of ICU studies with low and moderate risk of bias (Supplementary material 2, Table 7).
HA sepsis among all sepsis cases hospital-wide and in ICUs
Nine studies [6, 23, 24, 26, 28,29,30,31, 74] reported the proportion of HA sepsis among all sepsis patients (Fig. 4a; Supplementary material 2, Table 3) at the hospital level. The pooled proportion of HA sepsis was 23.6% (95% CI 17.0–31.8%) and ranged from 16.0 to 36.4% in individual studies. Among all patients with sepsis with organ dysfunction, the proportion of HA sepsis with organ dysfunction was 16.4% (95% CI 14.3–18.7%, range 11.3–32.9%) hospital-wide. Two studies [24, 30] showed that 25.4 and 35.0% cases of septic shock were hospital-acquired (pooled estimate: 31.7 [95% CI 23.4–41.4%]).
Pooled proportions of hospital-acquired sepsis cases among all sepsis cases. a Pooled proportions of hospital-acquired sepsis, sepsis with organ dysfunction or septic shock among hospital patients (including ICU wards) with sepsis, sepsis with organ dysfunction or septic shock. b Pooled proportions of ICU-acquired and hospital-acquired sepsis, sepsis with organ dysfunction and septic shock among ICU patients with sepsis, sepsis with organ dysfunction or septic shock. The “sepsis” group comprises studies among patients with sepsis, sepsis with organ dysfunction and septic shock. The “sepsis with organ dysfunction” group comprises studies among patients with sepsis with organ dysfunction and septic shock. HA hospital-acquired, ICU-A = ICU-acquired; 95% CI = 95% confidence interval
In ICUs, the pooled proportion of ICU-acquired sepsis among all sepsis patients was 31.4% (95% CI 24.9–38.8%) with individual study estimates ranging from 18.6 to 49.1% (Fig. 4b and Supplementary material 2, Table 3). The pooled proportion for ICU-acquired sepsis with organ dysfunction was 24.4% (95% CI 16.7–34.2%, range 10.3–42.5%). A pooled analysis of fourteen studies [32,33,34,35, 38, 41, 44,45,46, 48, 52, 54, 57] showed that 48.7% (95% 38.3–59.3%, range 18.7–69.4%) of all cases of sepsis with organ dysfunction treated in ICUs were hospital-acquired. For septic shock, two incidence studies [38, 50] showed that 35.7 and 37.4% of all septic shock cases treated in ICUs had a hospital origin (pooled estimate: 35.8% [95% CI 33.2–38.5%]). Pooled estimates were not different between studies with moderate and low risk of bias (Supplementary material 2, Table 7).
HA neonatal sepsis in NICUs
Nine studies [59,60,61, 64,65,66, 69,70,71] provided data on the incidence of HA neonatal sepsis in NICUs, expressed as cases per 1000 NICU-treated neonates. The pooled incidence of HA neonatal sepsis was 112.9 cases (95% CI 64.2–191.1%) per 1000 NICU-treated neonates (Fig. 5a; Table 1) with individual study estimates ranging from 18.4 to 368.2 cases per 1000 NICU-treated neonates. In NICUs, 56.6% (95% CI 43.5–68.8%, range 9.5–80.0%) of HAIs were found to be HA neonatal sepsis (Fig. 5b; Supplementary material 2, Table 4). The pooled estimate for blood culture-proven cases, a subgroup of HA neonatal sepsis cases, was 45.7 (95% CI 26.0–79.2, range 20.5–75.6) cases per 1000 NICU-treated neonates (five studies [59, 65, 66, 69, 70]) and accounted for 25.0% (95% CI 15.9–37.0%, range 16.5–50.7%)) of all HAIs.
Population-based incidence of HA sepsis
Only eight studies [12, 24, 31, 34, 44, 51, 54, 55] provided population-based incidence estimates of HA sepsis and HA sepsis with organ dysfunction, expressed as cases per 100,000 population per year. For hospital-treated HA sepsis, two studies from Spain [24] and China [31] reported incidences of 61.2 and 219.3 cases per 100,000 adult population per year (Table 1), respectively. The annual incidence of ICU-acquired sepsis was 5.8 and 12.7 cases per 100,000 population in two studies from Spain [24] and Italy [51], respectively. For ICU-treated HA sepsis with organ dysfunction, the pooled incidence was 40.8 (95% CI 14.3–116.9, range 13.8–175.0) cases per 100,000 population based on four studies [12, 34, 44, 54].
Mortality
No studies with data on attributable mortality of HA sepsis were identified. However, 19 studies [6, 12, 23, 26, 29, 30, 32,33,34,35, 37,38,39, 50,51,52,53, 57, 58] reported data on mortality among patients with HA sepsis. Hospital-wide (including ICUs) pooled mortality of HA sepsis and sepsis with organ dysfunction was 35.0% (95% CI 25.0–46.6%, range 24.5–54.6%) and 24.4% (95% CI 19.3–30.4%, range 19.2–30.0%), respectively (Supplementary material 2, Fig. 2A; Table 5). Only one study [30] reported the mortality of HA septic shock (52.5%).
Among ICU patients, mortality of ICU-acquired sepsis was 49.8% and 39.3% as shown by Sakr and colleagues [51] and Suka and colleagues [53], respectively (pooled estimate: 44.7% [95% CI 34.7–55.1%]) (Supplementary material 2, Fig. 2B; Table 5). Eight studies [32, 34, 35, 37, 39, 52, 57, 58] on ICU-acquired sepsis with organ dysfunction reported a pooled mortality of 40.5% (95% CI 30.6–51.2%) with individual study estimates ranging from 13.2 to 58.8%. Among ICU-treated patients with HA sepsis with organ dysfunction, including cases acquired in hospital wards and the ICU, the pooled mortality was 52.3% (95% CI 43.4–61.1%, range 30.1–64.6%) (seven studies [12, 33,34,35, 38, 52, 57]). All ICU-based studies reporting mortality data included only adult patients, except the study from Shime and colleagues [52]. Compared to the adult studies, Shime and colleagues showed lower mortality rates for ICU-acquired and HA sepsis with organ dysfunction in paediatric ICU patients (21.3% and 30.1%, respectively). The study by Quenot and colleagues [50] reported a mortality rate of 53.6% among ICU patients with HA septic shock. No study provided data on the mortality of neonates in NICUs with HA neonatal sepsis, but two studies [59, 68] reported a mortality of 10.0% and 38.0% for blood culture-proven neonatal sepsis, respectively (pooled estimate: 21.9% [95% CI 5.0–59.7%]) (Supplementary material 2, Table 5). Pooled mortality was not different between studies with moderate and low risk of bias (Supplementary material 2, Table 7).
Discussion
To our knowledge, this systematic review is the first to investigate the burden of HA sepsis at both hospital and ICU level. The main finding of our study is that HA sepsis poses a major burden among hospitalized patients, particularly in ICUs.
In ICUs, nearly one in four (24.4%) cases of sepsis with organ dysfunction was acquired during ICU stay, and more compelling, nearly half of all cases (48.7%) had originated in the hospital. The significance of HA sepsis with organ dysfunction in the ICU is also emphasized by our findings that 36 and 56 out of 1000 ICU patients developed sepsis with organ dysfunction in the ICU and in the hospital, respectively. This high rate has a major clinical implication as it has been shown that patients who develop sepsis during ICU stay have a significantly higher mortality and longer length of stay than ICU patients without sepsis [76]. Importantly, we found that the mortality of ICU patients with ICU- or hospital-acquired sepsis exceeded 40%, which is considerably higher than the overall mortality rates reported in critically ill ICU patients [77]. The incidence of HA sepsis was particularly high among neonates treated in NICUs. More than 110 out of 1000 admitted neonates suffered from HA sepsis, with HA neonatal sepsis representing more than 50% of all HAIs in this setting. Moreover, we found that the average length of ICU or hospital stay of patients with HA sepsis is much longer than that of patients with community-acquired sepsis, thus highlighting the considerable clinical and economic significance of HA sepsis in ICUs.
These findings indicate the urgent need to increase efforts to promote IPC programmes and interventions to reduce HAIs and their evolution to septic complications. WHO has repeatedly acknowledged the significant role of IPC programmes to combat sepsis, with clear calls to action [78]. Sepsis is avoidable in both the community and healthcare settings by preventing infection and halting its evolution to more severe conditions by rapidly establishing appropriate support and antimicrobial therapy [79, 80]. In particular, WHO and others have provided strong evidence and recommendations on the effectiveness of IPC to reduce the incidence of severe HAIs worldwide [13, 81,82,83,84], including sepsis [85]. However, much has still to be done, when considering that only 28% of countries worldwide report to have functional IPC programmes implemented at the national level and in all healthcare facilities, according to WHO recommendations [86].
Similar to a previous systematic review on the global incidence of hospital-treated sepsis [4], we identified a limited number of population-based studies. Thus, the global incidence of HA sepsis remains unclear and needs to be addressed in future studies. However, based on four large multicentre studies, we found a pooled population-level estimate of ICU-treated HA sepsis with organ dysfunction of 40.8 cases per 100,000 population per year. If the pooled estimate of the three European studies [34, 44, 54] (24.5 cases per 100,000 population) is extrapolated to countries of the European Union (EU) and European Economic Area (EEA) (518 million inhabitants), a tentative estimate would suggest approximately 127,000 cases of ICU-treated HA sepsis with organ dysfunction every year in this area. In line with this, Cassini and colleagues estimated that about 2,600,000 new cases of HAIs occur in the EU/EEA every year and that HAIs are considered the top infectious disease issue in this area [87].
Regarding the microbiological aetiology and related AMR patterns of HA sepsis, we could find limited evidence provided by seven studies only and substantial differences in findings were observed between individual ICU- and NICU-based studies. AMR is recognized as being one of the greatest public health challenges [88,89,90]. Accordingly, we found that a substantial proportion of organisms causing HA sepsis exhibited clinically relevant AMR. Given the clinical impact of resistant organisms on the treatment of sepsis [91], more studies specifying microbiological profiles in HA sepsis are needed.
Our study has some limitations. Due to the rigorousness of our methodology, we were only able to include a relatively low number of studies clearly reporting data on HA sepsis. Indeed, we excluded 490 papers, including some large good-quality studies, during the full-text review as the distinction between healthcare-associated and community-acquired sepsis was unclear. This is also linked to the fact that many epidemiological studies on sepsis rely on the use of ICD codes for sepsis case detection, rather than the prospective collection of data according to clinical consensus definitions, thus leading to a greater difficulty in distinguishing between healthcare-associated and community-acquired sepsis. Despite our broad search strategy with a special focus on low- and middle-income countries, most included hospital-wide and ICU-based studies were from high-income countries from the European and American WHO regions. Although our search/inclusion strategy comprised a wide range of languages, we cannot exclude that, due to language restrictions, some relevant studies particularly from low- and middle-income countries might have been missed. Therefore, similar to previous reviews or global estimates on sepsis, our findings might not represent the epidemiology of HA sepsis in low- and middle-income countries and in other WHO regions. However, it is likely that the incidence of HA sepsis among hospital-treated patients is even higher than our estimates suggest as HAIs are more prevalent in these countries [92]. Furthermore, we were unable to estimate the incidence of HA-sepsis-related deaths as no studies with data on attributable mortality were identified. Another limitation is that only two included studies used the current sepsis-3 consensus definition. The majority of hospital- and ICU-wide studies (including those that are recent) were based on the sepsis-1 definition. In view of this, our estimates for “sepsis with organ dysfunction”, including “severe sepsis” according to sepsis-1 and sepsis-2 definitions and “sepsis” according to the sepsis-3 definition, better reflect the current epidemiology of sepsis as the sepsis-3 definition includes organ dysfunction as defining criteria.
It is encouraging that the risk of bias of the individual ICU-based studies included was low to moderate, with the main source of risk being the unclear national representativeness of most reports. In contrast, the overall risk of bias was moderate to high in the great majority of hospital-wide and NICU-based studies, mostly due to low national representativeness and unclear reliability of the applied sepsis case definitions. We consistently found a very large heterogeneity between individual study estimates which should lead to caution in the interpretation of the meta-analyses results. However, based on our approach to only pool studies from similar settings, we decided that reporting these summaries provides a sufficiently robust analysis and a valuable contribution to this very relevant epidemiological topic. The variations between individual studies may be explained by methodological differences among studies, including applied sepsis case definitions, such as differences between clinical consensus definitions and administrative data [93, 94]. To our knowledge, there is no validated approach using administrative data to specifically identify sepsis cases of healthcare-associated origin. Indeed, it is a current research priority to reach a final international consensus on the most suitable ICD codes to trace sepsis cases and the most frequent conditions that lead to sepsis-related death. Moreover, as there is no validated sepsis definition for neonatal sepsis [95], case definitions varied and mainly relied on clinical symptoms and often did not include laboratory testing. In addition, the diagnostic criteria of neonatal sepsis used in the included studies might have also captured infections without any organ dysfunction. Furthermore, inter-study variations may also be caused by differences in patient characteristics (such as age [96] and comorbidities [97]), time of the study or could reflect true differences in the prevalence of underlying HAIs between countries and regions as well as between individual hospitals, as observed in several studies [10, 92, 98]. Ultimately, heterogeneity may be also explained by country differences in healthcare access and quality, since it has been shown that locations with less developed healthcare systems exhibit a higher sepsis incidence and mortality [3]. Based on these limitations and identified knowledge gaps, we conclude that more methodologically robust studies, especially from low- and middle-income countries, are needed to accurately understand the global burden of healthcare-associated sepsis (see Supplementary material 2, Table 8).
In summary, our study provides the first comprehensive summary of published evidence on the burden of HA sepsis including ICU-acquired sepsis. Our findings emphasize the public health importance of HA sepsis among hospitalized patients, with particular focus on ICUs, and the urgent need to improve the implementation of global and local IPC strategies to reduce the burden of HAIs, as well as approaches for their early diagnosis and adequate treatment to prevent a progression to sepsis complications. Further research is required to close major knowledge and methodological gaps identified by our study.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S (2017) recognizing sepsis as a global health priority—a WHO resolution. N Engl J Med 377(5):414–417. https://doi.org/10.1056/NEJMp1707170
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet 395(10219):200–211. https://doi.org/10.1016/S0140-6736(19)32989-7
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K (2016) Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 193(3):259–272. https://doi.org/10.1164/rccm.201504-0781oc
Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Namendys-Silva SA, Martin-Loeches I, Leone M, Lupu MN, Vincent JL (2018) Sepsis in intensive care unit patients: worldwide data from the intensive care over nations audit. Open Forum Infect Dis 5(12):ofy313. https://doi.org/10.1093/ofid/ofy313
Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M (2017) Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA 318(13):1241–1249. https://doi.org/10.1001/jama.2017.13836
Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N (2018) The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med 6(3):223–230. https://doi.org/10.1016/s2213-2600(18)30063-8
Saito H, Kilpatrick C, Pittet D (2018) The 2018 World Health Organization SAVE LIVES: clean your hands campaign targets sepsis in health care. Intensive Care Med 44(4):499–501. https://doi.org/10.1007/s00134-018-5097-9
World Health Organization (2011) Report on the burden of endemic health care-associated infection worldwide. World Health Organization, Geneva
Suetens C, Latour K, Karki T, Ricchizzi E, Kinross P, Moro ML, Jans B, Hopkins S, Hansen S, Lyytikainen O, Reilly J, Deptula A, Zingg W, Plachouras D, Monnet DL (2018) Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Eurosurveillance. https://doi.org/10.2807/1560-7917.es.2018.23.46.1800516
Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM, Beldavs Z, Gross C, Bamberg W, Sievers M, Concannon C, Buhr N, Warnke L, Maloney M, Ocampo V, Brooks J, Oyewumi T, Sharmin S, Richards K, Rainbow J, Samper M, Hancock EB, Leaptrot D, Scalise E, Badrun F, Phelps R, Edwards JR (2018) Changes in prevalence of health care-associated infections in U.S. Hospitals. N Engl J Med 379(18):1732–1744. https://doi.org/10.1056/nejmoa1801550
Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Angotti Carrara FS, Sousa JL, Caixeta N, Salomao R, Angus DC, Pontes Azevedo LC (2017) The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis 17(11):1180–1189. https://doi.org/10.1016/s1473-3099(17)30322-5
Schreiber PW, Sax H, Wolfensberger A, Clack L, Kuster SP (2018) The preventable proportion of healthcare-associated infections 2005–2016: systematic review and meta-analysis. Infect Control Hosp Epidemiol 39(11):1277–1295. https://doi.org/10.1017/ice.2018.183
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151 (4):264-269, w264. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101(6):1644–1655. https://doi.org/10.1378/chest.101.6.1644
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/sis international sepsis definitions conference. Crit Care Med 31(4):1250–1256. https://doi.org/10.1097/01.ccm.0000050454.01978.3b
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310. https://doi.org/10.1097/00003246-200107000-00002
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348(16):1546–1554. https://doi.org/10.1056/NEJMoa022139
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections. Am J Infect Control 16(3):128–140. https://doi.org/10.1016/0196-6553(88)90053-3
World Bank Group (2020). https://data.worldbank.org/. Accessed 16 Jan 2020
Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R (2012) Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 65(9):934–939. https://doi.org/10.1016/j.jclinepi.2011.11.014
Spencer FA, Iorio A, You J, Murad MH, Schunemann HJ, Vandvik PO, Crowther MA, Pottie K, Lang ES, Meerpohl JJ, Falck-Ytter Y, Alonso-Coello P, Guyatt GH (2012) Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ 345:e7401. https://doi.org/10.1136/bmj.e7401
Angkasekwinai N, Rattanaumpawan P, Thamlikitkul V (2009) Epidemiology of sepsis in Siriraj Hospital 2007. J Med Assoc Thail 92(Suppl 2):S68–S78
Esteban A, Frutos-Vivar F, Ferguson ND, Penuelas O, Lorente JA, Gordo F, Honrubia T, Algora A, Bustos A, Garcia G, Diaz-Reganon IR, de Luna RR (2007) Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med 35(5):1284–1289. https://doi.org/10.1097/01.ccm.0000260960.94300.de
Hagel S, Ludewig K, Frosinski J, Hutagalung R, Porzelius C, Gastmeier P, Harbarth S, Pletz MW, Brunkhorst FM (1946) Effectiveness of a hospital-wide educational programme for infection control to reduce the rate of health-care associated infections and related sepsis (ALERTS)—methods and interim results. Dtsch Med Wochenschr 138(34–35):1717–1722. https://doi.org/10.1055/s-0033-1349481
Jones SL, Ashton CM, Kiehne LB, Nicolas JC, Rose AL, Shirkey BA, Masud F, Wray NP (2016) Outcomes and resource use of sepsis-associated stays by presence on admission, Severity, and hospital type. Med Care 54(3):303–310. https://doi.org/10.1097/mlr.0000000000000481
Chaudhary NS, Donnelly JP, Wang HE, Puskarich M (2017) Racial disparities in severe sepsis in United States Academic Medical Centers. In: Paper presented at the 2017 Annual Meeting of the Society for Academic Emergency Medicine, Orlando, USA
Padro T, Smotherman C, Gautam S, Gerdik C, Fultz C, Lester D, Guirgis F (2018) Hospital-acquired sepsis: a descriptive study and comparison to communityacquired sepsis. Crit Care Med 46:1
Page DB, Donnelly JP, Wang HE (2015) Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the university healthsystem consortium. Crit Care Med 43(9):1945–1951. https://doi.org/10.1097/ccm.0000000000001164
Rodriguez F, Barrera L, De La Rosa G, Dennis R, Duenas C, Granados M, Londono D, Molina F, Ortiz G, Jaimes F (2011) The epidemiology of sepsis in Colombia: a prospective multicenter cohort study in ten university hospitals. Crit Care Med 39(7):1675–1682. https://doi.org/10.1097/CCM.0b013e318218a35e
Zhou J, Tian H, Du X, Xi X, An Y, Duan M, Weng L, Du B (2017) Population-based epidemiology of sepsis in a subdistrict of Beijing. Crit Care Med 45(7):1168–1176. https://doi.org/10.1097/ccm.0000000000002414
Adrie C, Alberti C, Chaix-Couturier C, Azoulay E, De Lassence A, Cohen Y, Meshaka P, Cheval C, Thuong M, Troche G, Garrouste-Orgeas M, Timsit JF (2005) Epidemiology and economic evaluation of severe sepsis in France: age, severity, infection site, and place of acquisition (community, hospital, or intensive care unit) as determinants of workload and cost. J Crit Care 20(1):46–58. https://doi.org/10.1016/j.jcrc.2004.10.005
Baharoon S, Telmesani A, Tamim H, Alsafi E, Aljohani S, Mahmoud E, Al-Jahdali H (2015) Community- versus nosocomial-acquired severe sepsis and septic shock in patients admitted to a tertiary intensive care in Saudi Arabia, etiology and outcome. J Infect Public Health 8(5):418–424. https://doi.org/10.1016/j.jiph.2014.12.003
Beovic B, Hladnik Z, Pozenel P, Siuka D (2008) Epidemiology of severe sepsis in Slovenian intensive care units for adults. J Chemother (Florence, Italy) 20(1):134–136. https://doi.org/10.1179/joc.2008.20.1.134
Blanco J, Muriel-Bombin A, Sagredo V, Taboada F, Gandia F, Tamayo L, Collado J, Garcia-Labattut A, Carriedo D, Valledor M, De Frutos M, Lopez MJ, Caballero A, Guerra J, Alvarez B, Mayo A, Villar J (2008) Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care (London, England) 12(6):R158. https://doi.org/10.1186/cc7157
Carvajal-Estupiñán JFN-JF, Ospina-Díaz JM (2016) Characterizing patients with diagnosis of sepsis in an intensive care unit, at Bucaramanga, Colombia 2010–2011. Arch Med (Manizales) 16(1):53
Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, Fang Q, Xu Q, Wang D, Jin Y, Yuan S, Wang J, Du Z, Sun Y, Fang X (2007) Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med 35(11):2538–2546. https://doi.org/10.1097/01.ccm.0000284492.30800.00
Dabar G, Harmouche C, Salameh P, Jaber BL, Jamaleddine G, Waked M, Yazbeck P (2015) Community- and healthcare-associated infections in critically ill patients: a multicenter cohort study. Int J Infect Dis 37:80–85. https://doi.org/10.1016/j.ijid.2015.05.024
Dougnac AL, Mercado MF, Cornejo RR, Cariaga MV, Hernandez GP, Andresen MH, Bugedo GT, Castillo LF (2007) Prevalence of severe sepsis in intensive care units. A national multicentric study. Rev Med Chil 135(5):620–630. https://doi.org/10.4067/s0034-98872007000500010
Gasparovic V, Gornik I, Ivanovic D (2006) Sepsis syndrome in Croatian intensive care units: piloting a national comparative clinical database. Croat Med J 47(3):404–409
SepNet Critical Care Trials Group (2016) Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med 42(12):1980–1989. https://doi.org/10.1007/s00134-016-4504-3
The Irish Critical Care Trials Group (2008) Intensive care for the adult population in Ireland: a multicentre study of intensive care population demographics. Crit Care (London, England) 12(5):R121. https://doi.org/10.1186/cc7018
Ribak S, Lazzeri S, Sosa L, Ojeda J (2008) Sepsis en una unidad de terapia intensiva polivalente: revisión de 2 años. Revista De La Facultad De Medicina De La Unne 27:12–15
Karlsson S, Varpula M, Ruokonen E, Pettila V, Parviainen I, Ala-Kokko TI, Kolho E, Rintala EM (2007) Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med 33(3):435–443. https://doi.org/10.1007/s00134-006-0504-z
Kubler A, Adamik B, Durek G, Mayzner-Zawadzka E, Gaszynski W, Karpel E, Duszynska W (2015) Results of the severe sepsis registry in intensive care units in Poland from 2003–2009. Anaesthesiol Intensive Ther 47(1):7–13. https://doi.org/10.5603/ait.2015.0002
Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent JL, Townsend S, Lemeshow S, Dellinger RP (2012) Outcomes of the surviving sepsis campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 12(12):919–924. https://doi.org/10.1016/s1473-3099(12)70239-6
Malacarne P, Langer M, Nascimben E, Moro ML, Giudici D, Lampati L, Bertolini G (2008) Building a continuous multicenter infection surveillance system in the intensive care unit: findings from the initial data set of 9,493 patients from 71 Italian intensive care units. Crit Care Med 36(4):1105–1113. https://doi.org/10.1097/CCM.0b013e318169ed30
Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, Longo CJ, Morrison T, Bentley D, Antman N (2009) A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis treatment and response registry. Crit Care Med 37(1):81–88. https://doi.org/10.1097/CCM.0b013e31819285f0
Ortiz G, Duenas C, Rodriguez F, Barrera L, de La Rosa G, Dennis R, Granados M, Londono D, Molina F, Jaimes F (2014) Epidemiology of sepsis in Colombian intensive care units. Biomedica: revista del Instituto Nacional de Salud 34(1):40–47. https://doi.org/10.1590/s0120-41572014000100007
Quenot JP, Pavon A, Binquet C, Kara F, Martinet O, Ganster F, Navellou JC, Castelain V, Barraud D, Cousson J, Poussel JF, Perez P, Kuteifan K, Noirot A (2012) Predictive and prognostic factors of septic shock of nosocomial origin. Crit Care 16:P49
Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, Fiore G, Filippini C, Ranieri VM (2013) Epidemiology and outcome of sepsis syndromes in Italian ICUs: a muticentre, observational cohort study in the region of Piedmont. Minerva Anestesiol 79(9):993–1002
Shime N, Kawasaki T, Saito O, Akamine Y, Toda Y, Takeuchi M, Sugimura H, Sakurai Y, Iijima M, Ueta I, Shimizu N, Nakagawa S (2012) Incidence and risk factors for mortality in paediatric severe sepsis: results from the national paediatric intensive care registry in Japan. Intensive Care Med 38(7):1191–1197. https://doi.org/10.1007/s00134-012-2550-z
Suka M, Yoshida K, Takezawa J (2006) Incidence and outcome of sepsis in Japanese intensive care units: the Japanese nosocomial infection surveillance system. Environ Health Prev Med 11(6):298–303. https://doi.org/10.1007/bf02898020
Vesteinsdottir E, Karason S, Sigurdsson SE, Gottfredsson M, Sigurdsson GH (2011) Severe sepsis and septic shock: a prospective population-based study in Icelandic intensive care units. Acta Anaesthesiol Scand 55(6):722–731. https://doi.org/10.1111/j.1399-6576.2011.02437.x
Vila Perez D, Jordan I, Esteban E, Garcia-Soler P, Murga V, Bonil V, Ortiz I, Flores C, Bustinza A, Cambra FJ (2014) Prognostic factors in pediatric sepsis study, from the Spanish Society of Pediatric Intensive Care. Pediatr Infect Dis J 33(2):152–157. https://doi.org/10.1097/01.inf.0000435502.36996.72
Vincent JL, Sakr Y (2013) The intensive care over nations (icon) audit: epidemiology of sepsis. In: Paper presented at the ESICM LIVES 2013 26th Annual Congress, Paris, France
Zahorec R, Firment J, Strakova J, Mikula J, Malik P, Novak I, Zeman J, Chlebo P (2005) Epidemiology of severe sepsis in intensive care units in the Slovak Republic. Infection 33(3):122–128. https://doi.org/10.1007/s15010-005-4019-2
Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma X, Ai Y, Xu Y, Liu D, An Y, Wu D, Sun R, Li S, Hu Z, Cao X, Zhou F, Jiang L, Lin J, Mao E, Qin T, He Z, Zhou L, Du B (2014) Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS ONE 9(9):e107181. https://doi.org/10.1371/journal.pone.0107181
Bas AY, Demirel N, Zenciroglu A, Gol N, Tanir G (2010) Nosocomial blood stream infections in a neonatal intensive care unit in Ankara, Turkey. Turk J Pediatr 52(5):464–470
Crivaro V, Bogdanovic L, Bagattini M, Iula VD, Catania M, Raimondi F, Triassi M, Zarrilli R (2015) Surveillance of healthcare-associated infections in a neonatal intensive care unit in Italy during 2006–2010. BMC Infect Dis 15:152. https://doi.org/10.1186/s12879-015-0909-9
Djordjevic ZM, Markovic-Denic L, Folic MM, Igrutinovic Z, Jankovic SM (2015) Health care-acquired infections in neonatal intensive care units: risk factors and etiology. Am J Infect Control 43(1):86–88. https://doi.org/10.1016/j.ajic.2014.10.005
Kamath S, Mallaya S, Shenoy S (2010) Nosocomial infections in neonatal intensive care units: profile, risk factor assessment and antibiogram. Indian J Pediatr 77(1):37–39. https://doi.org/10.1007/s12098-010-0005-5
Malla K, Malla T, Rao K (2013) Bacteriological profile of sepsis outbreak in the NICU of a tertiary care hospital in western Nepal. J Nepal Paediatr Soc. https://doi.org/10.3126/jnps.v33i1.7016
Mohammed D, El Seifi OS (2014) Bacterial nosocomial infections in neonatal intensive care unit, Zagazig University Hospital, Egypt. Egyptian Pediatr Assoc Gazette 62(3):72–79. https://doi.org/10.1016/j.epag.2014.10.001
Molina-Cabrillana J, Santana-Reyes C, Hernandez J, Lopez I, Dorta E (2006) Incidence of nosocomial infections at a neonatal intensive care unit: a six-year surveillance study. Enferm Infecc Microbiol Clin 24(5):307–312. https://doi.org/10.1157/13089665
Romanelli RM, Anchieta LM, Mourao MV, Campos FA, Loyola FC, Jesus LA, Armond GA, Clemente WT (2013) Notification of healthcare associated infections based on international criteria performed in a reference neonatal progressive care unity in Belo Horizonte, MG. Revista brasileira de epidemiologia 16(1):77–86
Sarvikivi E, Karki T, Lyytikainen O (2010) Repeated prevalence surveys of healthcare-associated infections in Finnish neonatal intensive care units. J Hosp Infect 76(2):156–160. https://doi.org/10.1016/j.jhin.2010.03.020
Shaw CK, Shaw P, Malla T, Malla KK (2012) The clinical spectrum and outcome of neonatal sepsis in a neonatal intensive care unit at a tertiary care hospital in western Nepal: January 2000 to December 2005—a retrospective study. Eastern J Med 17(3):119–125
Su BH, Hsieh HY, Chiu HY, Lin HC, Lin HC (2007) Nosocomial infection in a neonatal intensive care unit: a prospective study in Taiwan. Am J Infect Control 35(3):190–195. https://doi.org/10.1016/j.ajic.2006.07.004
Tavora AC, Castro AB, Militao MA, Girao JE, Ribeiro Kde C, Tavora LG (2008) Risk factors for nosocomial infection in a Brazilian neonatal intensive care unit. Braz J Infect Dis 12(1):75–79. https://doi.org/10.1590/s1413-86702008000100016
Trapani A, Dalbo K, Da Silva R, Sakae T, Silveira S, Feuerschuette O (2013) Incidence and epidemiology of nosocomial infection in a neonatal intensive care unit in southern Brazil. J. Perinat. Med. https://doi.org/10.1515/jpm-2013-2003
Vincent JLS, Y. (2013) The intensive care over nations (icon) audit: Epidemiology of sepsis. Paper presented at the ESICM LIVES 2013 26th Annual Congress, Paris, France
Goldstein B, Giroir B, Randolph A (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6(1):2–8. https://doi.org/10.1097/01.pcc.0000149131.72248.e6
M. CNSDJPWHEP (2017) Racial disparities in severe sepsis in United States Academic Medical Centers. In: Paper presented at the 2017 Annual Meeting of the Society for Academic Emergency Medicine, Orlando, USA
Irish Critical Care Trials Group (2008) Intensive care for the adult population in Ireland: a multicentre study of intensive care population demographics. Crit Care 12(5):R121. https://doi.org/10.1186/cc7018
Agodi A, Barchitta M, Auxilia F, Brusaferro S, D’Errico MM, Montagna MT, Pasquarella C, Tardivo S, Arrigoni C, Fabiani L, Laurenti P, Mattaliano AR, Orsi GB, Squeri R, Torregrossa MV, Mura I (2018) Epidemiology of intensive care unit-acquired sepsis in Italy: results of the SPIN-UTI network. Annali di igiene: medicina preventiva e di comunita 30(5 Supple 2):15–21. https://doi.org/10.7416/ai.2018.2247
Vincent J-L, Marshall J, Ñamendys-Silva S, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, Jimenez E, Sakr Y, Tomas E, Bibonge E, Charra B, Faroudy M, Doedens L, Farina Z, Adler D, Brealey D (2014) Assessment of the worldwide burden of critical illness: the Intensive Care Over Nations (ICON) audit. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(14)70061-X
Kilpatrick C, Saito H, Allegranzi B, Pittet D (2018) Preventing sepsis in health care—It’s in your hands: a World Health Organization call to action. J Infect Prev 19(3):104–106. https://doi.org/10.1177/1757177418769146
Pittet D, Allegranzi B (2018) Preventing sepsis in healthcare—200 years after the birth of Ignaz Semmelweis. Eurosurveillance. https://doi.org/10.2807/1560-7917.es.2018.23.18.18-00222
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34(6):1589–1596. https://doi.org/10.1097/01.ccm.0000217961.75225.e9
Alvarez-Moreno CA, Valderrama-Beltran SL, Rosenthal VD, Mojica-Carreno BE, Valderrama-Marquez IA, Matta-Cortes L, Gualtero-Trujillo SM, Rodriguez-Pena J, Linares-Miranda CJ, Gonzalez-Rubio AP, Vega-Galvis MC, Riano-Forero I, Ariza-Ayala BE, Garcia-Laverde G, Susmann O, Mancera-Paez O, Olarte N, Rendon-Campo LF, Astudillo Y, Trullo-Escobar MD, Orellano PW (2016) Multicenter study in Colombia: impact of a multidimensional International Nosocomial Infection Control Consortium (INICC) approach on central line-associated bloodstream infection rates. Am J Infect Control 44(11):e235–e241. https://doi.org/10.1016/j.ajic.2016.03.043
Rosenthal VD, Ramachandran B, Villamil-Gomez W, Armas-Ruiz A, Navoa-Ng JA, Matta-Cortes L, Pawar M, Nevzat-Yalcin A, Rodriguez-Ferrer M, Yildizdas RD, Menco A, Campuzano R, Villanueva VD, Rendon-Campo LF, Gupta A, Turhan O, Barahona-Guzman N, Horoz OO, Arrieta P, Brito JM, Tolentino MC, Astudillo Y, Saini N, Gunay N, Sarmiento-Villa G, Gumus E, Lagares-Guzman A, Dursun O (2012) Impact of a multidimensional infection control strategy on central line-associated bloodstream infection rates in pediatric intensive care units of five developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection 40(4):415–423. https://doi.org/10.1007/s15010-012-0246-5
Storr J, Twyman A, Zingg W, Damani N, Kilpatrick C, Reilly J, Price L, Egger M, Grayson ML, Kelley E, Allegranzi B (2017) Core components for effective infection prevention and control programmes: new WHO evidence-based recommendations. Antimicrob Resist Infect Control 6:6. https://doi.org/10.1186/s13756-016-0149-9
Price L, MacDonald J, Melone L, Howe T, Flowers P, Currie K, Curran E, Ness V, Waddell D, Manoukian S, McFarland A, Kilpatrick C, Storr J, Twyman A, Allegranzi B, Reilly J (2018) Effectiveness of national and subnational infection prevention and control interventions in high-income and upper-middle-income countries: a systematic review. Lancet Infect Dis 18(5):e159–e171. https://doi.org/10.1016/s1473-3099(17)30479-6
Hagel S, Ludewig K, Pletz MW, Frosinski J, Moeser A, Wolkewitz M, Gastmeier P, Harbarth S, Brunkhorst FM, Kesselmeier M, Scherag A (2019) Effectiveness of a hospital-wide infection control programme on the incidence of healthcare-associated infections and associated severe sepsis and septic shock: a prospective interventional study. Clin Microbiol Infect 25(4):462–468. https://doi.org/10.1016/j.cmi.2018.07.010
WHO WHO. https://www.who.int/antimicrobial-resistance/global-action-plan/database/en/. Accessed 16 Jan 2020
Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank HP, Ducomble T, Haller S, Harder T, Klingeberg A, Sixtensson M, Velasco E, Weiss B, Kramarz P, Monnet DL, Kretzschmar ME, Suetens C (2016) Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med 13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150
Tacconelli E, Pezzani MD (2019) Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis 19(1):4–6. https://doi.org/10.1016/s1473-3099(18)30648-0
Cassini A, Hogberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL (2019) Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 19(1):56–66. https://doi.org/10.1016/s1473-3099(18)30605-4
Jonas OB, Irwin A, Berthe FCJ, Le Gall FG, Marquez PV (2017) Drug-resistant infections: a threat to our economic future: final report (English), vol 2. World Bank Group, Washington, D.C.
Turnidge J (2003) Impact of antibiotic resistance on the treatment of sepsis. Scand J Infect Dis 35(9):677–682. https://doi.org/10.1080/00365540310015953
Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D (2011) Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet (London, England) 377(9761):228–241. https://doi.org/10.1016/s0140-6736(10)61458-4
Johnson AEW, Aboab J, Raffa JD, Pollard TJ, Deliberato RO, Celi LA, Stone DJ (2018) A comparative analysis of sepsis identification methods in an electronic database. Crit Care Med 46(4):494–499. https://doi.org/10.1097/ccm.0000000000002965
Mariansdatter SE, Eiset AH, Sogaard KK, Christiansen CF (2016) Differences in reported sepsis incidence according to study design: a literature review. BMC Med Res Methodol 16(1):137. https://doi.org/10.1186/s12874-016-0237-9
Schlapbach LJ, Kissoon N (2018) Defining pediatric sepsis. JAMA Pediatr 172(4):312–314. https://doi.org/10.1001/jamapediatrics.2017.5208
Lee SH, Hsu TC, Lee MG, Chao CC, Lee WC, Lai CC, Lee CC (2018) Nationwide trend of sepsis: a comparison among octogenarians, elderly, and young adults. Crit Care Med 46(6):926–934. https://doi.org/10.1097/ccm.0000000000003085
Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS (2006) The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med 34(10):2576–2582. https://doi.org/10.1097/01.ccm.0000239114.50519.0e
Russo PL, Stewardson AJ, Cheng AC, Bucknall T, Mitchell BG (2019) The prevalence of healthcare associated infections among adult inpatients at nineteen large Australian acute-care public hospitals: a point prevalence survey. Antimicrob Resist Infect Control 8:114. https://doi.org/10.1186/s13756-019-0570-y
ESICM LIVES 2019 Berlin, Germany. 28 September–2 October 2019 (2019) Intensive care medicine experimental 7(Suppl 3):55. https://doi.org/10.1186/s40635-019-0265-y
Acknowledgements
We thank Ilhamiyye Aliyeva, Ilona Enarovic and Simon Brinkwirth from the Robert Koch Institute for supporting data extraction. We also thank Rosemary Sudan for editing assistance.
Funding
The World Health Organization provided funding for this study and acted as a consultant in study design, data extraction and interpretation as well as in writing of the manuscript. The Federal Ministry of Health of Germany, the Federal Republic of Germany, also provided funding for this study.
Author information
Authors and Affiliations
Contributions
RM, HS, TH, ST, TE, AC and BA designed the study. RM, HS, TH and ST performed literature screening, study selection and data extraction. RM and TH assessed the risk of bias. RM conducted the statistical analyses. RM and BA led the writing of the manuscript. All authors revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
CFS received grants from the German Federal Ministry of Education and Research (BMBF) via the Center for Sepsis Control and Care (CSCC; FKZ: 01EO1002 and 01EO1502) and from the Innovation Funds of the German government (FKZ 01VSF17010). The other authors declare no competing interests.
Availability of data and materials
All data and materials generated during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors approved the final version submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer The opinions expressed in this article are those of the authors and do not reflect the official position of WHO. WHO takes no responsibility for the information provided or the views expressed in this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Markwart, R., Saito, H., Harder, T. et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med 46, 1536–1551 (2020). https://doi.org/10.1007/s00134-020-06106-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-06106-2