Abstract
Up to 10% of acute coronary syndromes are complicated by cardiogenic shock (CS) with contemporary mortality rates of 40–50%. The extent of ischemic myocardium has a profound impact on the initial, in-hospital, and post-discharge management and prognosis in this patient population. Individualized patient risk assessment plays an important role in determining appropriate revascularization, drug treatment with inotropes and vasopressors, mechanical circulatory support, intensive care support of other organ systems, hospital level of care triage, and allocation of clinical resources. This review will outline the underlying causes and diagnostic criteria, pathophysiology, and treatment of CS complicating acute coronary syndromes with a focus on (a) potential therapeutic issues from the perspective an interventional cardiologist, an emergency physician, and an intensive care physician, (b) the type of revascularization, and (c) new therapeutic advancements in pharmacologic and mechanical percutaneous circulatory support.
Similar content being viewed by others
Introduction
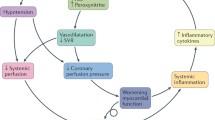
Cardiogenic shock (CS) as defined by the European Society of Cardiology (ESC) and the American Heart Association (AHA) is a state of critical end-organ hypoperfusion due to primary cardiac dysfunction [1,2,3]. Diagnostic criteria include hypotension (i.e., systolic blood pressure < 90 mmHg, or vasopressors required to achieve a blood pressure ≥ 90 mmHg), and signs of impaired organ perfusion (e.g., central nervous system abnormalities including confusion or lack of alertness, or even loss of consciousness; oliguria; cold, clammy skin and extremities, increased arterial lactate > 2 mmol/L) in the state of normovolemia or hypervolemia. Some clinical trials criteria also included hemodynamic parameters such as reduced cardiac index (CI, i.e., < 1.8 or < 2.2 L/min/m2 with cardiac support) or elevated left ventricular filling pressures (i.e., pulmonary capillary wedge pressure > 15 mmHg) [4, 9]. However, CS is a clinical diagnosis and does not require pulmonary artery catheterization. The clinical severity ranges from mild hypoperfusion to a pulseless state [5]. Of note, some described a “pre-shock state” that includes patients at risk for CS [6]. In addition, patients can have normotensive CS where hypoperfusion is present without hypotension. In a cohort of 49 patients, even in the presence of normal blood pressure, clinical signs of peripheral hypoperfusion, which may be subtle, are associated with a substantial risk of in-hospital death following acute myocardial infarction (AMI) [7]. The most severe form of CS is also named “refractory CS”, defined as a persisting shock despite the administration of volume, inotropes, and vasoconstrictors.
CS following acute myocardial infarction (AMI) has an incidence of 5–10% and is the leading cause of mortality in patients with AMI [8]. Half of the cases of CS are present at hospital admission and the other half develop following hospital admission [8]. Among patients with AMI, the SHOCK trial registry reported that predominant left ventricular failure (78.5%) was the most common etiology of CS, followed by severe mitral regurgitation (6.9%), ventricular septal rupture (VSR) (3.9%), right ventricular failure (2.8%), and cardiac tamponade (1.4%) [9].
Short-term mortality in CS complicating AMI is 40–60% and even more than 80% in case of ventricular septal rupture [9]. Mortality in CS is mostly seen in the intensive care unit (ICU) [10]. However, post-discharge mortality and symptomatic heart failure in CS patients are still higher than after AMI without CS [11].
Several clinical and biological factors have been used for prognosis assessment. Those factors have been recently regrouped into scores combining independent parameters—the Sleeper score (eight items; score from the SHOCK trial 2010) [12], the CardShock risk score (seven items as prior infarction and coronary artery bypass grafting are taken as one; 2015) [13], and the IABP-SHOCK II risk score (six items; 2017) (Table 1) [14]. Based on six variables with a maximum of 9 points there are three risk categories in the IABP-SHOCK II score. Patients in the low, intermediate, and high risk categories have an in-hospital mortality risk of 20–30%, 40–60%, and 70–90%, respectively. This prediction model is the first CS score with both internal and external validation [14].
Contemporary management of cardiogenic shock and AMI
CS patients may benefit as early as possible from prehospital management (Fig. 1), coronary revascularization, hemodynamic resuscitation and optimization, and the assessment and treatment of end-organ dysfunction. Notably, revascularization is the only well-studied evidence-based therapy with proven survival benefit. To provide benefit from this contemporary management of CS due to AMI, individual hospitals should be part of a regional network that includes a tertiary cardiogenic shock center.
Revascularization
As a result of its limited efficacy, fibrinolysis should be reserved for ST-elevation myocardial infarction (STEMI) patients when timely percutaneous coronary intervention (PCI) is not feasible [2]. The SHOCK trial is one of the milestone randomized trials in CS [15]. Although it failed to meet the primary endpoint—a reduction of 30-day mortality by an early revascularization-based management either with PCI or CABG—(46.7% vs 56.0%, p = 0.11) [15], there was a significant mortality reduction at 6 months and at long-term follow-up [16].
Since the widespread application of early revascularization in clinical practice, mainly influenced by a class I B guideline recommendation [2, 17], numerous registries have confirmed the survival advantage of early revascularization, leading to a subsequent reduction of CS mortality in the young and also the elderly [8, 18]. Real-world revascularization rates range from 27% to 54% in the USA [18], 47% in the GRACE registry [19], 70% in a Swiss registry [8], and 50% in a French registry [20]. This finding suggests that knowledge translation and implementation of early revascularization, despite the associated high risk, are a clinical priority.
Up to 85% of CS patients present with multivessel or left main coronary artery disease [21]. Patients presenting with multivessel coronary artery disease have higher mortality compared to patients with single-vessel disease. Current ESC STEMI guidelines encourage immediate multivessel PCI of all high-grade lesions, in addition to the culprit lesion with a class IIa C recommendation [2]. These recommendations are mainly based on pathophysiological considerations. The current evidence has recently been summarized in two meta-analyses showing an increased mortality at short-term follow-up with multivessel PCI and similar outcome at longer follow-up [21, 22]. Recently, the randomized, multicenter Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial showed a significant clinical benefit of a culprit-lesion-only strategy with a reduction in the primary endpoint of 30-day mortality or severe renal failure requiring renal replacement therapy (45.9% culprit-lesion-only PCI vs 55.4% immediate multivessel PCI group; relative risk 0.83; 95% confidence interval 0.71–0.96; p = 0.01) which was mainly driven by an absolute 8.2% reduction in 30-day mortality (43.3% vs 51.5%; relative risk 0.84; 95% confidence interval 0.72–0.98, p = 0.03) [23]. The CULPRIT-SHOCK results were consistent across all predefined subgroups. Thus, revascularization should be limited to culprit lesion only with possible staged revascularization. Intuitively, some angiographic subgroups, such as occluded right coronary artery culprit lesion with a concomitant high-grade proximal left anterior descending coronary artery or additional non-culprit subtotal lesions with Thrombolysis In Myocardial Infarction (TIMI) flow 1 or 2 may call for immediate multivessel PCI. However, this should be considered on an individual basis.
There may also be a role for emergent coronary artery bypass grafting; however, there is little evidence to guide surgical versus PCI revascularization. Current evidence from four observational reports, comparing PCI versus coronary artery bypass grafting (CABG), shows that the type of revascularization did not influence the outcome of CS patients [24, 25]. In current clinical practice, immediate CABG is only performed in less than 4% of patients [8, 26].
The ESC STEMI and NSTE-ACS guidelines recommend radial access (class 1 A) in stable patients if performed by experienced radial operators [2, 15,16,17]. In CS, the benefit of radial access is less evidence-based. A meta-analysis analyzing data of 8131 registry patients demonstrated that radial access was associated with a reduction in all-cause mortality as well as major adverse cardiac and cerebral events at 30-day follow-up in CS patients [27]. We propose to favor the radial access in case of experienced radial operators and in patients with a palpable radial pulse. Otherwise the femoral access may be chosen.
Platelet inhibitors and anticoagulation
Antithrombotic therapy including antiplatelets and anticoagulation is key during and after PCI. There are no specific trials in CS for oral antiplatelets; however, it is well known that in CS enteral resorption is impaired. Besides impaired enteral perfusion, mechanical ventilation (MV) with inability to swallow oral P2Y12 inhibitors plays a major role in the bioavailability of these drugs. Prasugrel/ticagrelor or clopidogrel in case of contraindications for the newer oral antiplatelets is indicated in addition to aspirin in all patients undergoing PCI. In intubated patients, crushed tablets need to be administered through a nasogastric tube. In stable infarction patients, crushed ticagrelor improved platelet inhibition in comparison to non-crushed tablets [28]. Because of the late and impaired onset of oral antiplatelets, glycoprotein IIb/IIIa inhibitors or cangrelor may be more liberally used in CS. During PCI, adjunctive anticoagulation including unfractionated heparin or low molecular weight heparin should be co-administered with antiplatelets. With a lack of specific randomized trials in CS the same recommendations apply as for other types of acute coronary syndrome [2, 17].
Mechanical complications
The incidence of infarct-related VSR without reperfusion ranged from 1% to 2% with a decrease to 0.2% in the era of reperfusion [29]. The median time from infarction to rupture is usually 24 h but may occur at up to 2 weeks. Without surgical repair of post-infarction VSR, 90% of patients die within 2 months [30]. Current mortality of surgical post-infarction VSR closure is as high as 50%. However, in two prospective registries, mortality rates were as high as 81–100% for patients with VSR and CS [29, 31]. Current guidelines recommend immediate surgical VSR closure, irrespective of the patient’s hemodynamic status, to avoid further hemodynamic deterioration [32]. Nevertheless, a subgroup of patients with VSR exists, for whom surgery is futile, because mortality approaches 100%; this includes the very elderly and patients with poor right ventricular function. As a result of the high mortality and suboptimal surgical results with a postoperative residual shunt found in up to 20% of treated patients, the technique of percutaneous VSR device closure has been developed. Though data are limited for post-infarction VSR interventional closure. A meta-analysis on all published reports with percutaneous VSR closure has recently been published showing similar mortality data compared to surgery [33].
In acute ischemic mitral regurgitation, only papillary muscle rupture needs immediate repair. Other causes, such as left ventricular global or regional remodeling or ischemic papillary muscle dysfunction, may resolve after revascularization and recovery of ventricular function. Accordingly, only 46% of the patients in the SHOCK trial registry underwent mitral valve surgery [34]. In contrast to VSR repair, surgery of papillary muscle rupture does not involve necrotic myocardium in suture lines. Therefore, mortality associated with this repair is lower [34]. The unpredictability of a rapid deterioration and death with papillary muscle rupture makes early surgery necessary.
Regional cardiogenic shock center network
Regional systems of care coupled with treatment algorithms have improved survival in MI. As recently suggested by AHA, applying a similar framework to CS management may lead to similar improvements in survival, and CS systems of care are emerging within existing regional cardiovascular emergency care networks (AHA guidelines) [3].
In a regionalized system of care for CS, individual hospitals would have CS treatment algorithms according to on-site capabilities, in relation to the leadership of regional “cardiac shock” center(s). In order to allow continuity in CS management, “cardiac shock” centers would require the creation of mobile multidisciplinary CS teams available 24 h a day, 7 days a week for on-site or off-site consultation, referral, and extracorporeal membrane oxygenation (ECMO)/mechanical circulatory support (MCS) insertion (Fig. 2). In addition, CS centers would have therapeutic technologies, including PCI and temporary MCS.
Algorithm of management of cardiogenic shock during the first period after cardiac shock center admission. VA-ECMO veno-arterial extracorporeal membrane oxygenation, BP blood pressure, CCU coronary care unit, ECG electrocardiography, IABP intra-aortic balloon pump, ICU intensive care unit, LV left ventricle, MV mechanical ventilation, NE norepinephrine, PAC pulmonary artery catheter, PCI percutaneous coronary intervention, RRT renal replacement therapy, RV right ventricle
Hemodynamic management in the ICU
Recognition of CS should trigger a swift assessment of the etiology and hemodynamic profile based upon history, physical examination, laboratory investigations, electrocardiogram (ECG), and echocardiography. Although initial assessment and management do not require invasive hemodynamic monitoring, Table 2 provides an overview of the main indications for advanced hemodynamic monitoring.
Arterial line
Arterial blood pressure should be monitored using a continuously transduced arterial line and more advanced invasive hemodynamic monitoring may be considered in severe refractory cases and/or when mechanical complications supervene. The initial target mean arterial blood pressure should be in the range of 60–65 mmHg. However, this blood pressure target has not been validated in randomized clinical trials [35]. An arterial line also allows regular blood gas analysis and arterial lactate monitoring. Devices based on arterial waveform analysis through proprietary algorithms have been developed to measure a number of hemodynamic parameters, including cardiac output. Despite their widespread use, a number of studies have shown inconsistent performance of these devices in the setting of acute/very low cardiac output states and CS [36].
Central venous catheter
The central venous waveform allows visualization of an elevated pressure (in the context of respiratory support), or abnormal waveform should trigger further evaluation of both heart and lungs/ventilatory parameters. The insertion of a central venous catheter also allows one to analyze ScvO2 for the assessment of the ratio of global oxygen demand and supply, hence response to therapy. The central line also represents the preferential route for the administration of inotropes and/or vasopressors [37]. Finally, central line trajectory monitoring may help to estimate congestion of extrathoracic organs such as the kidneys as it directly translates to output pressure of intra-abdominal organs. Hence, all efforts should be made to keep the central venous pressure < 12 mmHg.
Echocardiography
In the context of CS, transthoracic echocardiography has strengths and weaknesses. Indeed, echocardiography is recommended repeatedly during ICU stay for evaluation of left and right ventricular function, valve dysfunction, and exclusion/diagnosis of mechanical complications.
Echocardiography is also emerging as a hemodynamic monitoring tool in ICUs to estimate cardiac output, cardiac filling pressures, predict volume responsiveness, and determine response to critical care interventions [38]. Its use in monitoring patients with CS, however, can prove challenging. First, there are a number of intensive care interventions that may fundamentally alter echocardiographic findings, and every study must be interpreted in the pharmacopathological context. Second, few parameters validated in either the outpatient or general cardiology setting have been validated in CS complicating AMI. Third, unlike invasive monitoring, echocardiography cannot measure intracardiac pressures and merely provides an estimate of pressure differences between different chambers. The intelligent application of physiological echocardiography (i.e., stroke distance, pulmonary vascular resistance, cardiac electromechanics including ventricular–ventricular interactions and heart–lung/ventilator interactions), interpreted in the clinical context can, however, be used to monitor and guide ongoing interventions, including requirement for pacing interventions, vasoactive drug therapies, and ventilatory settings [39]. Further, echocardiography is mandatory for the use of mechanical circulatory support in order to assess contraindications, inform the type and level of support required, guide and monitor institution of support, assess complications, and predict potential for weaning [39]. Ultrasound also provides information on lung congestion and pleural effusion.
Pulmonary artery catheter (PAC)
Randomized studies and several meta-analyses have failed to confirm a clinical benefit of the PAC in a wide range of critically ill patient pathologies [40,41,42]. Current recommendations of the European Society of Intensive Care Medicine still consider PAC as a useful tool in some patients with severe CS, especially in case of right ventricular dysfunction or CS unresponsive to initial therapies, reflecting standard practice in expert centers in the management of this condition [13, 35].
Laboratory testing
In case of CS related to AMI we recommend performing full laboratory testing, ideally twice a day, until restoration of stable hemodynamic parameters. Laboratory testing can indicate in the first hours the extent of organ injury and the prognosis of the patients while serial measures give information on the aggravation or recovery of organ functions. Laboratory testing should include troponin, natriuretic peptides (and ST2 for prognosis [43]), lactate (mostly in the first days [44]), renal, liver, and basic coagulation function tests as well as blood count.
Inotropes and vasopressors
Hemodynamic alteration in CS complicating AMI includes cardiac impairment with or without low vascular resistance [45]. In addition to timely revascularization, vasopressors and/or inotropes are required to restore systemic perfusion in the prehospital setting, in the catheterization laboratory, or in the ICU. In general, inotropes and vasopressors should be used at the lowest dose and the shortest time possible. Furthermore, these agents have different effects at different doses making interpretation of the evidence even more difficult. In addition, we suggest that clinicians integrate clinical, laboratory, and hemodynamic variables to determine response to therapy to determine appropriate vasoactive drug dosing and titration based on clinical, laboratory, and hemodynamic multimodal monitoring.
When blood pressure needs to be rapidly restored, norepinephrine may be a reasonable first-line agent. Various studies showed that norepinephrine is safer than dopamine [44], vasopressin [46], or epinephrine, with a lower risk of atrial arrhythmias [43].
Given the reduced cardiac output in CS, the addition of an inotropic agent may help to improve stroke volume after hemodynamic stabilization with an inopressor. In case of evidence of predominant low cardiac output and preserved perfusion pressure, dobutamine is the initial therapy and (starting dose 2.5 µg/kg/min) may act rapidly to restore stroke volume.
Concerning inotropic agents associated with vasodilator properties, levosimendan may also be used in particular in patients on chronic beta-blocker therapy given its inotropic effect being independent of beta-adrenergic stimulation [1]. However, further studies are needed.
The following agents are generally not considered first-line therapies in CS: (1) Dopamine was shown to be associated with increased 28-day mortality as compared to norepinephrine, although this effect may be explained by chance [44]. In the same study, dopamine also showed a higher number of arrhythmias [44]. (2) Epinephrine led to higher lactate levels in a small randomized trial in CS [47, 48]. This is supported by a retrospective analysis of the Cardshock cohort revealing that epinephrine use was associated with higher short-term mortality [43]. A prospective double-blind multicenter study confirmed detrimental the effect of epinephrine on outcome in CS patients [49]. (3) Vasopressin is also not preferred because this drug did not change cardiac power index and cardiac index while norepinephrine increased it [46].
Mechanical circulatory support
Recommendations on the use of intra-aortic balloon pump (IABP) and extracorporeal life support in CS will be described below. A consensus nomenclature of various extracorporeal life supports was recently described [50].
Intra-aortic balloon pump
The IABP-SHOCK II randomized 600 patients with CS complicating AMI and early revascularization to IABP or conventional treatment and found no difference in the primary study endpoint of 30-day mortality between the two treatment groups [26]. The results of the primary study endpoint were confirmed by a lack of beneficial effects for any of the secondary study endpoints and also through longer follow-up [26, 51]. These results led to a downgrading of the IABP in the ESC guidelines with a current class IIIB recommendation for the routine use of the IABP in CS [2, 17]. The 2017 ESC STEMI guidelines now recommend IABP consideration only in patients with mechanical complications (class IIa, level C) [2].
Percutaneous active mechanical circulatory support devices
Partly a result of the lack of benefit of IABP, active MCS are increasingly used [52, 53]. However, the current evidence to support the routine use of MCS is limited [54]. Current devices, mode of action, and evidence regarding percutaneous MCS for treatment in CS have been summarized previously [5]. Table 3 gives an overview over current devices and technical features.
Current devices include the TandemHeart™ (Cardiac Assist, Inc, Pittsburgh, US) which removes arterialized blood from the LA and returns it to the lower abdominal aorta or iliac arteries, via a femoral artery cannula, with retrograde perfusion of the abdominal and thoracic aorta. Another percutaneous device is the Impella® 2.5, CP, or 5.0 (Abiomed Europe, Aachen, Germany) which is placed across the aortic valve, using the femoral access, either percutaneously or by surgical cut-down.
In the recent IMPRESS-in-Severe-SHOCK trial 48 patients with STEMI associated CS requiring MV were randomized to Impella CP versus IABP [55]. The 30-day mortality primary endpoint was based on a power calculation with non-realistic mortality rates and thus this trial is markedly underpowered. Not surprisingly, there was no difference in the primary endpoint of all-cause mortality after 30 days; however, the lack of benefit in any of the other parameters including arterial lactate may be a concern with respect to the efficacy of the device [56]. A trial adequately powered for patients with CS is required before routine therapy with this device can be recommended.
A most recent meta-analysis including the IMPRESS-in-Severe-SHOCK trial showed no difference in mortality for the 148 included patients. There was some improvement in arterial lactate and also MAP. On the other hand there were significantly more bleeding complications [54]. Some registry data suggest a benefit for MCS insertion before revascularization [57, 58]. However, these data need to be confirmed in randomized trials.
VA-ECMO
Extracorporeal circulatory support (ECLS) with veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is another potential first-line device in CS since it provides both respiratory and cardiac support [59]. VA-ECMO bypasses the heart and/or the lung owing to extraction of blood from a venous inflow cannula and return to the arterial system via an outflow cannula after decarboxylation and oxygenation [60]. It may be the preferred option in cases of CS because of a few major advantages: (1) the possibility of rapid application, (2) the applicability in case of malignant arrhythmia due to lack of alteration of flow condition, and (3) rapid improvement in oxygenation. On the other hand, VA-ECMO carries a relevant risk of thromboembolic events and limb ischemia. It increases left ventricle afterload, which may impair heart function recovery and aggravate hydrostatic pulmonary edema [61]. Furthermore, it requires a specialist team to insert and run ECMO such as perfusionists or ECMO specialists. Strategies combining IABP or IMPELLA and VA-ECMO to decrease LV pressures have suggested a clinical benefit which should be evaluated in specific trials [62, 63]. In a recent meta-analysis based on observational registry data, VA-ECMO was associated with a 33% higher 30-day survival compared with IABP (95% confidence interval 14–52%; p < 0.001) but no difference when compared with TandemHeart/Impella (− 3%; 95% confidence interval − 21% to 14%; p = 0.70) [64].
Taken together, although MCS are theoretically appealing devices that may interrupt the vicious spiral of ischemia, hypotension, and myocardial dysfunction and allow for the recovery of ischemic myocardium, the extracorporeal support and contact with artificial surfaces of these devices might further promote the systemic inflammatory response. A second potentially deleterious effect is severe bleeding.
Currently, percutaneous MCS should be restricted to the use in refractory CS (class of recommendation II b C) and will rely on individual experience in dedicated centers for selected patients [2]. Additional randomized trials are needed for a more complete assessment of the role of different circulatory supportive strategies in CS [65].
Management of organ dysfunction (Fig. 2)
Respiratory distress
Chest X-ray at admission allows one to assess pulmonary congestion, cardiac size, and the position of endotracheal tube and supportive devices (pacing wires and MCS).
CS related to LV failure is usually complicated by pulmonary edema associated with impaired gas exchange [66]. Metabolic acidosis increases the compensatory respiratory load. Most CS patients may need respiratory support to provide adequate gas exchange and to relieve the work of breathing. The majority of guidelines and reviews recommend invasive ventilation in CS, and its use ranges from 60% to 80%. In isolated right ventricular failure, whether those patients are mechanically ventilated or on non-invasive ventilation (NIV), caution is advised because of the undesirable effect of positive end-expiratory pressure on right ventricular afterload and function [66]. In few patients with acute pulmonary edema and mild metabolic and hemodynamic alterations, NIV can be installed and seems a safe option [67].
Acute kidney injury
The incidence of acute kidney injury (AKI)—defined by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria—is roughly one-third in CS patients [61]. Increased creatinine levels and anuria—but not oliguria—was associated with increased mortality. The development of AKI seemed at least partly associated with persistent decrease in CI and MAP and elevated central venous pressure. In case of AKI, we recommend a rapid restoration of renal perfusion pressure and avoiding the use of nephrotoxic agents. Data supporting those recommendations are needed.
Acute liver injury
Overall, elevated liver parameters can be interpreted as generally poor hemodynamic status. Liver function tests are altered in over 50% of patients suffering from CS [68]. Elevated alanine transaminase (ALT) and aspartate transaminase (AST) may be interpreted as a direct sign of liver hypoperfusion, associated with increased mortality. Hemodynamics should be stabilized as in renal dysfunction.
Brain injury due to resuscitated cardiac arrest
Registries and trials have reported a 29–41% incidence of cardiac arrest in patients with CS following MI [19, 69,70,71]. The optimal brain management of these patients has not been well studied. The European Resuscitation Council and the European Society of Intensive Care Medicine guidelines for post-resuscitation care suggest multiparametric monitoring of hemodynamics (blood pressure, cardiac index, central venous oxygen saturation, lactate), oxygenation, and ventilation, to avoid secondary brain injury [72]. Benefits of the optimization of cerebral blood flow using transcranial Doppler and/or cerebral NIRS should be evaluated in further studies. Targeted temperature management (TTM) has been shown to improve neurologically intact survival in cardiac arrest patients [73, 74], though data in CS following cardiac arrest are missing. The ongoing HYPO-ECMO study (NCT02754193) will evaluate the effect of moderate hypothermia and normothermia in CS patients treated with VA-ECMO. Meanwhile, we may recommend, if possible, TTM for 24 h in patients with MI associated with CS and an out-of-hospital cardiac arrest who remain unresponsive. By contrast, in patients in CS and no cardiac arrest, there is insufficient data to support the use of TTM (Fig. 3).
Structured approach to neuroprognostication (from [72] with authorization)
Right ventricular infarction
Acute right ventricular (RV) infarction usually occurs in relation to acute inferior wall MI caused by occlusion of the proximal right coronary artery [75]. It is an independent, strongly age-dependent predictor of short-term mortality in patients with inferior MI. The anatomic occlusion of the infarct-related artery and functional impairment of the right ventricle are poorly correlated. Unlike the left ventricle, the right ventricle may remain viable for days after an infarct. Therefore, late reperfusion is an option that may be considered in patients with inferior MI complicated by RV dysfunction. Despite younger age, less multivessel disease, and better left ventricular ejection fraction, prognosis is no different in CS patients with or without acute RV failure [76].
Future directions
Future directions address many aspects of CS management. An overview is provided in Table 4.
Conclusion
Despite early revascularization the mortality of patients with CS is still high. In cases where CS has developed, we advocate for multidisciplinary care in a specialized center. Patients who are treated according to clinical practice guidelines, with early reperfusion for all patients and an optimal supportive intensive care treatment, have a mortality rate of approximately 40%, as shown in recent randomized trials.
Currently, there are many unresolved issues, such as the access site for reperfusion, type of reperfusion (culprit-lesion-only PCI with staged revascularization versus immediate CABG in severe coronary artery disease), the optimal inotrope or vasopressor support, the role and potential treatment options of concomitant inflammation, the selection and timing of patients for MCS, optimal MV strategy, treatment of bleeding complications, and among many others. Some of these open questions may be addressed by ongoing trials.
In general, randomized controlled trials in CS are difficult to perform and are often more costly than trials in other clinical conditions because of the complexity of the studies. Therefore, many believe that conducting a randomized study in this critically ill population is still not possible; however, identifying interventions that improve survival in this high morbidity and mortality condition is likely to have major public health implications and should therefore be thoroughly tested.
Abbreviations
- AHA:
-
American Heart Association
- AKI:
-
Acute kidney injury
- ALT:
-
Alanine aminotransferase
- AMI:
-
Acute myocardial infarction
- AST:
-
Aspartate aminotransferase
- BP:
-
Blood pressure
- CABG:
-
Coronary artery bypass graft
- CCU:
-
Coronary care unit
- CI:
-
Cardiac index
- CVP:
-
Central venous pressure
- CS:
-
Cardiogenic shock
- ECG:
-
Electrocardiography
- ECLS:
-
Extracorporeal circulatory life support
- ECMO:
-
Extracorporeal membrane oxygenation
- ERC:
-
European Resuscitation Council
- ESC:
-
European Society of Cardiology
- ESICM:
-
European Society of Intensive Care Medicine
- IABP:
-
Intra-aortic balloon pump
- ICU:
-
Intensive care unit
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- LV:
-
Left ventricle
- MAP:
-
Mean arterial pressure
- MCS:
-
Mechanical circulatory support
- MV:
-
Mechanical ventilation
- NE:
-
Norepinephrine
- NIV:
-
Non-invasive ventilation
- NSTE-ACS:
-
Non-ST-segment elevation acute coronary syndrome
- OHCA:
-
Out-of-hospital cardiac arrest
- PAC:
-
Pulmonary artery catheter
- PCI:
-
Percutaneous coronary intervention
- RCT:
-
Randomized controlled trial
- RRT:
-
Renal replacement therapy
- RV:
-
Right ventricle
- ST2:
-
Suppression of tumorigenicity 2
- STEMI:
-
ST-elevation myocardial infarction
- TIMI:
-
Thrombolysis In Myocardial Infarction
- TTM:
-
Targeted temperature management
- VA-ECMO:
-
Veno-arterial extracorporeal membrane oxygenation
- VSD:
-
Ventricular septal defect
- VSR:
-
Ventricular septal rupture
References
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA et al (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18(8):891–975
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S et al (2018) 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177
van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK et al (2017) Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 136(16):e232–e268
Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, Cecconi M, Choi DJ, Cohen Solal A, Christ M et al (2016) Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med 42(2):147–163
Thiele H, Ohman EM, Desch S, Eitel I, de Waha S (2015) Management of cardiogenic shock. Eur Heart J 36(20):1223–1230
Furer A, Wessler J, Burkhoff D (2017) Hemodynamics of cardiogenic shock. Interv Cardiol Clin 6(3):359–371
Menon V, Slater JN, White HD, Sleeper LA, Cocke T, Hochman JS (2000) Acute myocardial infarction complicated by systemic hypoperfusion without hypotension: report of the SHOCK trial registry. Am J Med 108(5):374–380
Jeger RV, Radovanovic D, Hunziker PR, Pfisterer ME, Stauffer JC, Erne P, Urban P (2008) Ten-year incidence and treatment of cardiogenic shock. Ann Intern Med 149:618–626
Hochman JS et al (2000) Cardiogenic shock complicating acute myocardial infarction--etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol 36(3 Suppl A):1063–1070
Zannad F, Mebazaa A, Juilliere Y, Cohen-Solal A, Guize L, Alla F, Rouge P, Blin P, Barlet MH, Paolozzi L et al (2006) Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: the EFICA study. Eur J Heart Fail 8(7):697–705
Shah RU, de Lemos JA, Wang TY, Chen AY, Thomas L, Sutton NR, Fang JC, Scirica BM, Henry TD, Granger CB (2016) Post-hospital outcomes of patients with acute myocardial infarction with cardiogenic shock: findings from the NCDR. J Am Coll Cardiol 67(7):739–747
Sleeper LA, Reynolds HR, White HD, Webb JG, Dzavik V, Hochman JS (2010) A severity scoring system for risk assessment of patients with cardiogenic shock: a report from the SHOCK trial and registry. Am Heart J 160(3):443–450
Harjola VP, Lassus J, Sionis A, Kober L, Tarvasmaki T, Spinar J, Parissis J, Banaszewski M, Silva-Cardoso J, Carubelli V et al (2015) Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 17(5):501–509
Poss J, Koster J, Fuernau G, Eitel I, de Waha S, Ouarrak T, Lassus J, Harjola VP, Zeymer U, Thiele H et al (2017) Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 69(15):1913–1920
Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J et al (1999) Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 341(9):625–634
Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward PE, Col J, White HD (2006) Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA 295:2511–2515
Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP et al (2016) 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 37(3):267–315
Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J (2009) Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 119(9):1211–1219
Awad HH, Anderson FA Jr, Gore JM, Goodman SG, Goldberg RJ (2012) Cardiogenic shock complicating acute coronary syndromes: insights from the Global Registry of Acute Coronary Events. Am Heart J 163(6):963–971
Aissaoui N, Puymirat E, Tabone X, Charbonnier B, Schiele F, Lefevre T, Durand E, Blanchard D, Simon T, Cambou J-P et al (2012) Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J 33(20):2535–2543
de Waha S, Jobs A, Pöss J, Stiermaier T, Fuernau G, Eitel I, Zeymer U, Desch S, Thiele H (2018) Multivessel versus culprit lesion only percutaneous coronary intervention in cardiogenic shock complicating acute myocardial infarction: a systematic review and meta-analysis. Eur Heart J 7:28–37
Kolte D, Sardar P, Khera S, Zeymer U, Thiele H, Hochadel M, Radovanovic D, Erne P, Hambraeus K, James S et al (2017) Culprit vessel only versus multivessel percutaneous coronary intervention in patients with cardiogenic shock complicating ST-elevation myocardial infarction: a collaborative meta-analysis. Circulation 10(10):e005582
Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C et al (2017) PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 377:2419–2432
Mehta RH, Lopes RD, Ballotta A, Frigiola A, Sketch MH Jr, Bossone E, Bates ER (2010) Percutaneous coronary intervention or coronary artery bypass surgery for cardiogenic shock and multivessel coronary artery disease? Am Heart J 159(1):141–147
White HD, Assmann SF, Sanborn TA, Jacobs AK, Webb JG, Sleeper LA, Wong C-K, Stewart JT, Aylward PEG, Wong S-C et al (2005) Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock: results from the should we emergently revascularize occluded coronaries for cardiogenic shock (SHOCK) trial. Circulation 112(13):1992–2001
Thiele H, Zeymer U, Neumann F-J, Ferenc M, Olbrich H-G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G et al (2012) Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367(14):1287–1296
Pancholy SB et al (2015) Impact of access site choice on outcomes of patients with cardiogenic shock undergoing percutaneous coronary intervention: A systematic review and meta-analysis. Am Heart J 170(2):353–361
Parodi G, Xanthopoulou I, Bellandi B, Valenti R, Gkizas V, Migliorini A, Karanikas S, Abbate R, Antoniucci D, Alexopoulos D (1030) Ticagrelor crushed tablets administration in STEMI patients: the Mashed Or Just Integral Tablets of ticagrelOr (MOJITO) study. Eur Heart J 2014(Abstract Supplement):35
Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS, Vahanian A, Califf RM, Topol EJ (2000) Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation 101(1):27–32
Lee WY, Cardon L, Slodki SJ (1962) Perforation of infarcted interventricular septum. Report of a case with prolonged survival and review of the literature. Arch Intern Med 109:731–735
Menon V, Webb JG, Hillis LD, Sleeper LA, Abboud R, Dzavik V, Slater JN, Forman R, Monrad ES, Talley JD et al (2000) Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol 36(3 Suppl A):1110–1116
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P et al (2014) 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 35(37):2541–2619
Schlotter F, de Waha S, Eitel I, Desch S, Fuernau G, Thiele H (2016) Interventional post myocardial infarction ventricular septal defect closure: systematic review of current evidence. EuroIntervention 12:94–102
Thompson CR, Buller CE, Sleeper LA, Antonelli TA, Webb JG, Jaber WA, Abel JG, Hochman JS (2000) Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK trial registry. J Am Coll Cardiol 36(3, Supplement 1):1104–1109
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL et al (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40(12):1795–1815
Thiele RH, Bartels K, Gan TJ (2015) Cardiac output monitoring: a contemporary assessment and review. Crit Care Med 43(1):177–185
Ricard JD, Salomon L, Boyer A, Thiery G, Meybeck A, Roy C, Pasquet B, Le Miere E, Dreyfuss D (2013) Central or peripheral catheters for initial venous access of ICU patients: a randomized controlled trial. Crit Care Med 41(9):2108–2115
De Backer D (2014) Ultrasonic evaluation of the heart. Curr Opin Crit Care 20(3):309–314
Price S, Platz E, Cullen L, Tavazzi G, Christ M, Cowie MR, Maisel AS, Masip J, Miro O, McMurray JJ et al (2017) Expert consensus document: echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol 14(7):427–440
Shah MR, Hasselblad V, Stevenson LW, Binanay C, O’Connor CM, Sopko G, Califf RM (2005) Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 294(13):1664–1670
Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 366(9484):472–477
Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW (2005) Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 294(13):1625–1633
Tarvasmaki T, Lassus J, Varpula M, Sionis A, Sund R, Kober L, Spinar J, Parissis J, Banaszewski M, Silva Cardoso J et al (2016) Current real-life use of vasopressors and inotropes in cardiogenic shock—adrenaline use is associated with excess organ injury and mortality. Crit Care 20(1):208
De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL et al (2010) Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 362(9):779–789
Hochman JS (2003) Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation 107(24):2998–3002
Jolly S, Newton G, Horlick E, Seidelin PH, Ross HJ, Husain M, Dzavik V (2005) Effect of vasopressin on hemodynamics in patients with refractory cardiogenic shock complicating acute myocardial infarction. Am J Cardiol 96(12):1617–1620
Levy B, Perez P, Perny J, Thivilier C, Gerard A (2011) Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med 39:450–455
Levy BC-JR, Legras A, Morichau- Beauchant T, Leone M, Ganster F, Jean-Pierre Quenot JP, Kimmoun A, Cariou A, Lassus J, Harjola VP, Meziani F, Louis G, Rossignol P, Duarte K, Girerd N, Mebazaa A, Vignon Ph (2018) Epinephrine versus norepinephrine in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol (in press)
Levy B, Meziani F, Leone M, Guiot P, Quenot JP, Louis G, Legras A, Duarte K, Vignon P (2018) Comparison of epinephrine and norepinephrine for the treatment of cardiogenic shock following acute myocardial infarction. OPTIMA CC study. Ann Intensive Care 8(Suppl 1):CO-65
Conrad SA, Broman LM, Taccone FS, Lorusso R, Malfertheiner MV, Pappalardo F, Di Nardo M, Belliato M, Grazioli L, Barbaro RP et al (2018) The Extracorporeal Life Support Organization Maastricht Treaty for nomenclature in extracorporeal life support. A position paper of the Extracorporeal Life Support Organization. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.201710-2130CP
Thiele H, Zeymer U, Neumann F-J, Ferenc M, Olbrich H-G, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K et al (2013) Intraaortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock. Final 12-month results of the randomised IntraAortic Balloon Pump in cardiogenic shock II (IABP-SHOCK II) trial. Lancet 382:1638–1645
Sandhu A, McCoy LA, Negi SI, Hameed I, Atri P, Al’Aref SJ, Curtis J, McNulty E, Anderson HV, Shroff A et al (2015) Utilization of mechanical circulatory support in patients undergoing percutaneous coronary intervention: insights from the NCDR. Circulation 132:1243–1251
Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, Agrawal S, Arora S, Patel N, Wald J et al (2018) Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol 107(4):287–303
Thiele H, Jobs A, Ouweneel DM, Henriques JPS, Seyfarth M, Desch S, Eitel I, Pöss J, Fuernau G, de Waha S (2017) Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 38:3523–3531
Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis M, Wykrzykowska JJ, Koch KT, Baan J et al (2017) Impella CP versus intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock. The IMPRESS in Severe Shock trial. J Am Coll Cardiol 69:278–287
Zeymer U, Thiele H (2017) Mechanical support for cardiogenic shock—lost in translation? J Am Coll Cardiol 69:288–290
Basir MB, Schreiber T, Dixon S, Alaswad K, Patel K, Almany S, Khandelwal A, Hanson I, George A, Ashbrook M et al (2018) Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv 91(3):454–461
Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, Khandelwal AK, Ohman EM, O’Neill WW (2017) Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol 119(6):845–851
Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Schmidt M, Mastroianni C, Chastre J, Leprince P et al (2016) The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 42(3):370–378
Abrams D, Garan AR, Abdelbary A, Bacchetta M, Bartlett RH, Beck J, Belohlavek J, Chen YS, Fan E, Ferguson ND et al (2018) Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med (in press)
Aissaoui N, Caudron J, Leprince P, Fagon JY, Lebreton G, Combes A, Diebold B (2017) Right-left ventricular interdependence: a promising predictor of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 43(4):592–594
Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Mullerleile K, Colombo A et al (2017) Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 19(3):404–412
Aso S, Matsui H, Fushimi K, Yasunaga H (2016) The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med 44(11):1974–1979
Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engstrom AE, Lagrand WK, Cherpanath TG, Driessen AH, de Mol BA, Henriques JP (2016) Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med 42(12):1922–1934
Combes A, Brodie D, Chen YS, Fan E, Henriques JPS, Hodgson C, Lepper PM, Leprince P, Maekawa K, Muller T et al (2017) The ICM research agenda on extracorporeal life support. Intensive Care Med 43(9):1306–1318
Masip J, Peacock WF, Price S, Cullen L, Martin-Sanchez FJ, Seferovic P, Maisel AS, Miro O, Filippatos G, Vrints C et al (2018) Indications and practical approach to non-invasive ventilation in acute heart failure. Eur Heart J 39(1):17–25
Hongisto M, Lassus J, Tarvasmaki T, Sionis A, Tolppanen H, Lindholm MG, Banaszewski M, Parissis J, Spinar J, Silva-Cardoso J et al (2017) Use of noninvasive and invasive mechanical ventilation in cardiogenic shock: a prospective multicenter study. Int J Cardiol 230:191–197
Jantti T, Tarvasmaki T, Harjola VP, Parissis J, Pulkki K, Sionis A, Silva-Cardoso J, Kober L, Banaszewski M, Spinar J et al (2017) Frequency and prognostic significance of abnormal liver function tests in patients with cardiogenic shock. Am J Cardiol 120(7):1090–1097
Ostenfeld S, Lindholm MG, Kjaergaard J, Bro-Jeppesen J, Moller JE, Wanscher M, Hassager C (2015) Prognostic implication of out-of-hospital cardiac arrest in patients with cardiogenic shock and acute myocardial infarction. Resuscitation 87:57–62
Urban P, Stauffer JC, Bleed D, Khatchatrian N, Amann W, Bertel O, van den Brand M, Danchin N, Kaufmann U, Meier B et al (1999) A randomized evaluation of early revascularization to treat shock complicating acute myocardial infarction. The (Swiss) Multicenter Trial of Angioplasty for Shock-(S)MASH. Eur Heart J 20(14):1030–1038
Fordyce CB, Wang TY, Chen AY, Thomas L, Granger CB, Scirica BM, Henry TD, Wong GC, Ramanathan K, Hansen CM et al (2016) Long-term post-discharge risks in older survivors of myocardial infarction with and without out-of-hospital cardiac arrest. J Am Coll Cardiol 67(17):1981–1990
Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C (2015) European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post-resuscitation care 2015: section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 95:202–222
Bernard SA, Smith K, Finn J, Hein C, Grantham H, Bray JE, Deasy C, Stephenson M, Williams TA, Straney LD et al (2016) Induction of therapeutic hypothermia during out-of-hospital cardiac arrest using a rapid infusion of cold saline: the RINSE trial (rapid infusion of cold normal saline). Circulation 134(11):797–805
Testori C, Sterz F, Holzer M, Losert H, Arrich J, Herkner H, Krizanac D, Wallmuller C, Stratil P, Schober A et al (2012) The beneficial effect of mild therapeutic hypothermia depends on the time of complete circulatory standstill in patients with cardiac arrest. Resuscitation 83(5):596–601
Harjola VP, Mebazaa A, Celutkiene J, Bettex D, Bueno H, Chioncel O, Crespo-Leiro MG, Falk V, Filippatos G, Gibbs S et al (2016) Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail 18(3):226–241
Jacobs AK, Leopold JA, Bates E, Mendes LA, Sleeper LA, White H, Davidoff R, Boland J, Modur S, Forman R et al (2003) Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol 41(8):1273–1279
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
AM received lecture fees from Novartis, Orion, and Abbott, research grants from Roche, and consultant fees from Servier and Sanofi. GL received speaker fees from Orion, Abbvie, and Tenax (companies producing or commercializing levosimendan). BL received lecture fees from Pulsion, Baxter, Orion, and Lilly, and consultant fees from Novartis, Orion, and Baxter. Other coauthors have no conflicts to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mebazaa, A., Combes, A., van Diepen, S. et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med 44, 760–773 (2018). https://doi.org/10.1007/s00134-018-5214-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5214-9