Purpose
To review the available knowledge related to the use of ECCO2R as adjuvant strategy to mechanical ventilation (MV) in various clinical settings of acute respiratory failure (ARF).
Methods
Expert opinion and review of the literature.
Results
ECCO2R may be a promising adjuvant therapeutic strategy for the management of patients with severe exacerbations of COPD and for the achievement of protective or ultra-protective ventilation in patients with ARDS without life-threatening hypoxemia. Given the observational nature of most of the available clinical data and differences in technical features and performances of current devices, the balance of risks and benefits for or against ECCO2R in such patient populations remains unclear
Conclusions
ECCO2R is currently an experimental technique rather than an accepted therapeutic strategy in ARF—its safety and efficacy require confirmation in clinical trials.
Similar content being viewed by others
Introduction
Extracorporeal carbon dioxide removal (ECCO2R) is a technique providing artificial respiratory support by removal of CO2 from blood through an extracorporeal gas exchanger, and is a feature of several strategies of extracorporeal life support, including venovenous (VV) and arteriovenous (AV) extracorporeal membrane oxygenation [1, 2]. However, low flow VV devices which provide CO2 removal but not oxygenation are emerging as a potential respiratory support strategy [3–7]. Although originally developed as a means to improve the respiratory management of patients with ARDS [8], advances in technology and a better knowledge of the technique have enabled its use in other clinical syndromes, such as severe asthma or decompensated chronic obstructive pulmonary disease (COPD) and as a bridge to transplantation [9–11]. This review article will summarize the available knowledge related to the use of ECCO2R as adjuvant strategy to mechanical ventilation (MV) in various clinical settings of acute respiratory failure (ARF).
The detrimental consequences of hypercapnia
ECCO2R is applied to avoid excessive hypercapnia and its detrimental effects resulting from the pathophysiological changes of the respiratory system or from specific MV protective strategies (permissive hypercapnia).
Severe hypercapnia may negatively affect extrapulmonary organ function, particularly the brain and the cardiovascular system. By increasing cerebral blood flow, hypercapnia elevates intracranial pressure [12]. Hypercapnic acidosis increases pulmonary vasoconstriction and, in addition to microvascular alterations and to the effects of positive-pressure MV, dramatically increases right ventricular (RV) afterload [13]. At the same time, hypercapnia and hypercapnic acidosis decrease myocardial contractility. This altered hemodynamic profile contributes to RV-arterial decoupling and acute RV dysfunction [13, 14].
However, the clinical implications of hypercapnia and hypercapnic acidosis on the lung have not been fully elucidated. It has been hypothesized that hypercapnia may attenuate pulmonary inflammation by affecting several pathways. Experimental models have reported that hypercapnia reduces the production of superoxide as well as other free radical compounds and decreases the release of tumor necrosis factor α (TNF-α) and interleukin-1 (IL-1) from macrophages. Furthermore, hypercapnic acidosis attenuates lung injury by reducing inflammation via inhibition of NF-κB activity [14]. These data support the hypothesis that hypercapnic acidosis may attenuate lung and other organ injury in septic lung injury [14]. However, these beneficial effects may be the result of systemic acidosis rather than hypercapnia per se, as buffering the pH worsened lung injury [15]. Conversely, hypercapnic acidosis may contribute to lung injury by increasing both the production of nitric oxide and inflammation, and impair alveolar epithelial cell function by causing the endocytosis of Na+/K+ ATPase. Finally, due to its immunosuppressive properties, hypercapnic acidosis may worsen lung injury by exacerbating pulmonary bacterial infection [14]. Given the contradictory nature of these findings, further research is needed to clarify the relationship between hypercapnic acidosis and such complex pathophysiological pathways.
Principles of the technique
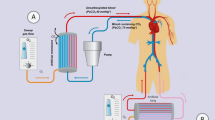
ECCO2R devices include a drainage cannula placed in a central vein (VV systems) or artery (AV systems), an artificial lung, and a return cannula into the venous system (Fig. 1) [16–18]. Early roller or peristaltic pumps have been now replaced by centrifugal or diagonal pumps with radial rotating impellers which generate the driving pressure with lower blood trauma [3, 16–18]. Pumpless devices can be used only in the AV configuration. However, a mean arterial pressure of at least 70 mmHg or an arteriovenous pressure gradient ≥60 mmHg are required to guarantee a sufficient blood flow in the circuit, and a cardiac index higher than 3 L/min/m2 has to be maintained, as a proportion of cardiac output which passes through the ECCO2R does not affect peripheral perfusion [3, 16–19]. The presence of hemodynamic instability and/or heart failure that often characterize critically ill patients may therefore limit the use of such devices. Advances in technology, design, and materials in newer ECCO2R systems have allowed a reduction in the degree of anticoagulation required to maintain the performance of these devices. During ECCO2R, the application high sweep fresh gas flow generates a diffusion gradient which allows CO2 removal (Fig. 2) [16–18]. In addition, it depends on the blood flow to the membrane: 1 L of blood contains around 500 mL of CO2 or more and the CO2 production per minute is about 200–250 mL/min, thus a blood flow of 0.5 L/min would be sufficient to remove all of the CO2 produced by the body [3, 16–18]. Accordingly, ECCO2R systems can now provide clinically meaningful levels of CO2 removal with relatively low blood flow (300–1000 mL/min), although CO2 removal may be enhanced with higher blood flow (i.e., “mid-flow” of 1000–2000 mL/min) [65]. Although it has been reported that a blood flow of 300–500 mL/min potentially replaces about 50% or more of the exchange function of the native lung [3, 16–18], the percentage is very often lower, as it depends on the actual blood CO2 content, hemoglobin concentration and the exchange performance of the membrane. Therefore, ECCO2R may typically remove about 25% of total CO2 production [3, 16–18]. Recent experimental investigations have focused on enhancing the efficiency in CO2 removal by acidification of the extracorporeal blood in animal models and electrodialysis with promising results [20, 21].
The choice of the vascular access and the type of cannulas depend on the configuration of the circuit. The AV configuration usually requires two single-lumen wire-reinforced femoral arterial and venous cannulas which enable the drainage and the return of blood, respectively. As the heart is the driving pump in the AV configuration, the cannulas should be large enough to reduce resistance to blood flow [16–19]. Conversely, the presence of pumps in the newer VV ECCO2R systems enables the use of dual-lumen catheters, with blood flows between 300 and 1500 mL/min.
Potential clinical indications for ECCO2R
The current evidence regarding the use of ECCO2R in various forms of ARF remains limited, with most of the data coming from small case series and observational studies conducted in expert centers.
Role of ECCO2R in ARDS patients
Mechanical ventilation, the mainstay treatment for ARDS, carries the risk of several adverse effects, the most important being ventilator-induced lung injury (VILI) as a consequence of an inhomogeneous lung overdistension. This secondary lung injury contributes to increasing the release of inflammatory mediators, which may negatively affect extra-pulmonary organ function [22]. Lung-protective ventilatory strategies have been demonstrated to improve patient outcomes [22–24]. Nevertheless, recent studies have shown that, even after applying protective MV, lung injury may still occur [22–26]. Further reductions in tidal volume may therefore limit VILI, potentially decreasing mortality [22]. The potential benefits of limiting VILI further have resulted in a growing interest in ECCO2R as an adjuvant strategy in patients with ARDS (Table 1) [16].
ECCO2R was first proposed as a response to the failure of the NIH-funded RCT of ECMO in adults with severe ARF [27]. Differences in MV between treated and control groups were primarily limited to changes in the FiO2. This is due to the fact that, in the early 1970s, MV was set to optimize oxygenation by increasing airway pressures, tidal volumes, and FiO2, and oxygen toxicity was thought at the time as the most relevant damaging factor associated with MV. The potential for VILI was largely unrecognized and ignored.
At that time, however, Gattinoni and Kolobow worked on ECCO2R [28]. Control of CO2 by the artificial lung allowed complete control of ventilation, either spontaneous or mechanical, providing lung rest. The evolving conceptual paradigm, therefore, was to use extracorporeal support to rest the lung in order to avoid VILI, which was hypothesized but not yet confirmed [29]. Well before the benefits of lung protective ventilation could be shown in clinical trials, the application of ECCO2R in ARDS patients was effective in decreasing barotrauma in small clinical series [29, 30]. ECCO2R with blood flow in the range of 1.5–2.5 L/min was coupled to low-frequency, low-pressure ventilation with higher PEEP levels and lower driving pressure and respiratory rate. With these settings, the native lung only provides some oxygenation, while the artificial lung removes CO2 and supports the remaining oxygenation needs.
As a mean to simplify the technique, AV pumpless techniques were proposed to provide ECCO2R [31]. But it was only when low-resistance capillary membrane lungs became available that AV ECCO2R became popular, though initially proposed as a mean to provide oxygenation rather than as a lung-protective adjunct [32]. Later, Terragni et al. showed that up to a third of ARDS patients were at still at risk of VILI despite being ventilated according to the ARDSNet protocol (<6 mL/kg PBW) [33]. In an attempt to reduce VILI, they used ECCO2R (blood flow 300 mL/min) to decrease tidal volume to less than 4 mL/kg PBW, while maintaining normal pH and PaCO2 and without any patient-related complications. Of note, the reduction in ventilator intensity was associated with a decrease in bronchoalveolar inflammatory cytokines [34]. Similar results were reported in 15 patients with moderate ARDS, in which ECCO2R (blood flow of 435 mL/min) provided through a 15.5-Fr dual lumen venous catheter allowed a reduction of tidal volume from 6.2 to 4 mL/kg PBW. ECCO2R was effective in correcting pH and PaCO2 but two patients required escalation to ECMO because of life-threatening hypoxemia [6].
Bein et al. conducted a randomized trial in ARDS patients comparing the effects of a 3-mL/kg PBW facilitated by AV-ECCO2R with a 6-mL/kg PBW ventilatory strategy [35]. Due to the small sample size, there was no significant difference in the primary outcome of ventilator-free days, but a post hoc analysis demonstrated shorter MV duration in patients with severe hypoxemia (PaO2/FiO2 <150 mmHg) treated with ECCO2R. In addition to the possibility of further reducing tidal volume, ECCO2R could also be helpful in maintaining the conventional protective ventilation of 6 mL/kg PBW. For instance, Moss et al. [36] noticed a sustained reduction in peak inspiratory pressures following the commencement of ECCO2R in nine ARDS patients. Finally, a systematic review of 14 studies (495 patients) confirmed that ECCO2R is feasible, facilitates the use of lower tidal volume ventilation, and was associated with an increased number of ventilator-free days but not improved survival [37].
Though still largely an experimental technique, ECCO2R appears to be a promising adjunct to ventilatory management in patients with ARDS that has the potential to minimize VILI [38]. More information will be available from the results of an ongoing international multicenter pilot study (SUPERNOVA; ClinicalTrials.gov NCT02282657) to assess the safety and feasibility of MV at 4 mL/kg PBW (facilitated by ECCO2R), and a UK multicenter RCT comparing ECCO2R to enable lower tidal volume ventilation versus standard care (REST; ClinicalTrials.gov NCT02654327).
Role of ECCO2R in chronic obstructive pulmonary disease (COPD)
Noninvasive ventilation (NIV) is the standard of care of acute hypercapnic respiratory failure (AHRF) that may fail in almost 40% of the most severe forms leading to endotracheal intubation and invasive MV (IMV) [39], which is associated with high mortality [40]. Notably, the mortality for patients who require IMV after NIV failure has been demonstrated to be higher than those who receive IMV at the beginning of treatment. As a result, ECCO2R may be a good therapeutic option for patients with AHRF to prevent failure of NIV and avoid IMV (Table 2). In fact, the use of ECCO2R in patients with AHRF may enhance the efficacy of CO2 washout of NIV, therefore lowering respiratory rate, dynamic hyperinflation, and intrinsic PEEP. Importantly, by avoiding IMV and thus endotracheal intubation, it is also possible to limit the adverse effects related to analgo-sedation which include hemodynamic derangement, prolonged weaning, and a range of neurological disorders when sedation is prolonged in time. In addition, the absence of analgo-sedation allows the patients to drink, to eat, to communicate with familiars, to receive aerosolized medications, and to perform active physiotherapy. Furthermore, it has been recently demonstrated that ECCO2R, by decreasing respiratory rates, may be effective in reducing the work of breathing and in lowering CO2 production of the respiratory muscles. This in turn contributes to the decrease of PaCO2 [41]. As result, it may facilitate the withdrawal of IMV and may favor early endotracheal extubation.
ECCO2R to obviate the need for IMV, prior to resolution of the COPD exacerbation
Kluge et al. [42] investigated the feasibility of an AV pumpless extracorporeal lung-assist (PECLA) device in 21 COPD patients who did not respond to NIV. The use of PECLA was associated with a decrease in PaCO2 levels and improved pH after 24 h, and obviated the need for intubation and IMV in 90% of treated patients. The retrospective analysis with a control group showed no significant difference in mortality at 28 days (19% with ECCO2R vs. 24% without ECCO2R) or 6 months (both groups 33%) and in the median ICU or hospital length of stay (15 vs. 30 days and 23 vs. 42 days, respectively). In the study by Burki et al. [43] 20 hypercapnic patients with COPD were treated with ECCO2R using a 15.5-Fr dual-lumen catheter allowing a mean blood flow of 430 mL/min. The authors reported an improvement in both hypercapnia and respiratory acidosis, and IMV was avoided in all nine patients receiving NIV. More recently, Del Sorbo et al. [7], reported that ECCO2R with a 14-Fr dual-lumen catheter and blood flow rates of 177–333 mL/min not only improved respiratory acidosis but also reduced the need for intubation in 25 COPD patients at high risk of NIV failure. Compared to a matched group of historical controls, the risk of being intubated and hospital mortality were significantly lower in the ECCO2R group. These results were challenged in a recent investigation by Braune et al. [44], which showed that IMV was avoided in 56% of cases treated with ECCO2R, but was associated with a higher incidence of complications. However, several differences have been highlighted between these two studies, including the inclusion of patients with relative contraindications to NIV and the unexpected high incidence of hypoxemic patients [45]. Finally, Morelli et al. [46] confirmed the efficacy of ECCO2R (with a flow rate of 250–450 mL/min through a 13-Fr dual-lumen catheter) in reducing in PaCO2 in series of 30 patients with acute hypercapnic respiratory failure due to exacerbation of COPD, who refused endotracheal intubation after failing NIV. The duration of ECCO2R was 2–16 days, and it was possible to prevent endotracheal intubation in 27 patients.
ECCO2R to facilitate the weaning from mechanical ventilation
In the report from Elliot et al. [47], the addition of a pumpless ECCO2R to IMV in two patients suffering from life-threathening asthma, corrected hypercapnia and acidosis, allowed the reduction of other supportive measures and the favored the weaning from mechanical ventilation. In the study by Burki et al. [43], in the subgroup of 11 patients receiving IMV, ECCO2R allowed the weaning from the mechanical ventilator in only 3 patients. Nevertheless, although not fully weaned, another 3 patients reduced the ventilator support. By using a dual-lumen cannula (20–23 Fr) with blood flow rates of 1–1.7 L/min, Abrams et al. [48] successfully weaned and extubated five COPD patients with acute respiratory acidosis requiring IMV within 24 h, and ambulated them within 48 h of ECCO2R support. All patients survived to hospital discharge. Likewise, using a pediatric VV ECMO system (with blood flow rates of 0.9 L/min through a 19 Fr dual-lumen cannula placed in right internal jugular vein) in two adult patients with a COPD exacerbation, Roncon-Albuquerque Jr [49] reported early extubation after 72 h and patient mobilization out of bed at day 6. A retrospective data analysis from the reports of 12 patients with hypercapnic respiratory failure confirms the efficacy of ECCO2R in correcting hypercapnia and reducing both ventilation pressures and minute volumes at median blood flow rates of 1.2–1.4 L/min. Among the investigated patients, six suffering from primarily hypercapnic lung failure due to obstructive lung disease or fibrosis were rapidly weaned from the system and survived to hospital discharge. Of note, five patients were awake and breathing spontaneously during ECCO2R [50]. Taken together, these findings support the notion that ECCO2R may be helpful in avoiding intubation during NIV and in facilitating weaning from MV.
Nevertheless, the observational nature of available data makes it impossible to understand the efficacy and safety of such strategies in these patients. Therefore, multicenter RCTs to evaluate the efficacy of ECCO2R to improve long-term outcomes in COPD patients are needed. Currently, there are a number of ongoing studies of ECCO2R in AHRF patients (ClinicalTrials.gov. NCT02260583; NCT02107222; NCT02259335).
Role of ECCO2R in the bridge to lung trasplantation
It is well recognized that the patients who develop an acute deterioration of gas exchanges requiring IMV while waiting for lung transplantation are more prone to die when compared with those patients who do not require IMV [66]. The rational of using ECCO2R in such patients lies in the possibility to avoid endotracheal intubation and IMV and thus in limiting their adverse effects (e.g., ventilator-associated pneumonia) which may preclude transplantion. In addition, by using ECCO2R, it is possible to avoid analgo-sedation, allowing the patient to maintain respiratory muscle tone and to perform active physiotherapy. Despite this rational, reports regarding the use of ECCO2R in this particular subgroup of hypercapnic patients are still scarce. Schellongowski et al. [51] performed a retrospective study investigating 20 patients suffering from bronchiolitis obliterans syndrome, cystic fibrosis, and idiopathic pulmonary fibrosis with the indication to primary lung transplantation (n = 13) or lung re-trasplantation (n = 7). The use of VV and pumpless AV ECCO2R was associated with an improvement in both hypercapnia and acidosis within the first 12 h of application. After a bridging period ranging from 4 to 11 days, 19 patients (95%) were successfully transplanted; hospital survival was 75%. A very recent pooled data analysis confirmed that patients supported with ECCO2R before lung retransplantation had a trend toward a better survival [52]. In the light of these findings, ECCO2R may be even helpful in thoracic surgical procedures other than lung transplantation [53]. Nevertheless, given the complexity and the challenging clinical conditions of the patients waiting for lung transplantation, the use of ECCO2R in these patients should only be performed in expert centers.
ECCO2R-related complications
Although ECCO2R seems to be effective in improving gas exchanges, in mitigating hypercapnic acidosis and possibly in reducing the rate of endotracheal intubation, its use may have pulmonary and hemodynamic consequences and it can be associated to adverse events. Adverse events include patient-related events, circuit placement events and mechanical events (Table 3).
In four ARDS studies in which tidal volume was reduced from 6 to 4 and 3 mL/kg PBW [6, 34–36], the use of ECCO2R was associated with the need for higher FiO2 due to compensating for the decreased mean airway pressure, low ventilation–perfusion ratio (both promote atelectasis), and a lower partial pressure of alveolar oxygen secondary to a decreased lung respiratory quotient [54–56]. In addition, higher levels of positive end-expiratory pressure (PEEP) were also required to maintain lung recruitment and functional residual capacity [6, 34–36]. Nevertheless, the need for higher PEEP to counteract the decrease of pulmonary vascular resistance induced by the reduction of hypercapnia should be considered. Of note, in the study from Fanelli et al., 40% of the patients developed life-threatening hypoxemia and required either extracorporeal membrane oxygenation (ECMO) or the prone position [6]. Likewise, in the ÉCLAIR study, 28% of the patients treated with ECCO2R required endotracheal intubation due to progressive hypoxemia [44]. The worsening of hypoxemia during ECCO2R may be due to: (1) the clinical course of the respiratory failure (evolution of infiltrates, presence of abundant respiratory secretions, atelectasis); and (2) excessive CO2 removal which leads, especially during spontaneous ventilation, to the reduction of tidal volume with increased risk of atelectasis and lower partial pressure of alveolar oxygen (decreased lung respiratory quotient) [45]. The risk of worsening hypoxemia has therefore to be considered a possible drawback of ECCO2R.
Due to the low blood flow required, the newer ECCO2R systems do not negatively affect systemic hemodynamics. In this regard, it has been reported that ECCO2R decreases pulmonary hypertension and unloads RV, leading to improved RV-arterial coupling [57–59]. As both ARDS and COPD patients are at high risk of RV dysfunction, besides improving gas exchange ECCO2R may contribute to prevent acute right ventricular dysfunction [60]. None of the available reports or studies showed increased vasopressors requirements during ECCO2R. Improved myocardial performance and possibly reduced level of sedation may account for these findings. Forsteret al. [61] demonstrated in ten patients that a combined ECCO2R and renal-replacement circuit decreased vasopressor requirements. Whether this decrease was mainly the effect of renal-replacement therapy in reducing systemic acidosis remains to be determined.
Major adverse events can be caused by vein and/or arterial cannulation, with increased risk depending on the choice of vascular access, and the type and the size of cannulas. Bein et al. [35] reported transient ischemia of the lower limb in one patient and ‘false’ aneurysm in other two patients after placing a 15-Fr cannula in the femoral artery. One perforation of the left iliac vein with death secondary to retroperitoneal bleeding occurred in the study of Burki et al. [43]. Retroperitoneal hematoma and vein perforation at catheter insertion have also been reported [45].
The low blood flow adopted by newer ECCO2R devices increases the risk of catheter and membrane thrombosis. Anticoagulation protocols with heparin are therefore required to maintain the ECCO2R efficiency and performance [62]. The occurrence of minor bleeding events can be considered the most frequent complication, and can be the consequence of anticoagulation or catheter insertion. Although such minor bleeding events seem not to affect the hemodynamics or outcome, they may be associated with a higher number of units of red blood cells transfused during the treatment [6, 35, 42–45]. Major bleeding episodes (bleeding episodes requiring more than two blood transfusions) were observed in patients from the larger case series [6, 42, 43, 45]. Notably, in the very recent study by Braune et al. [44], 11 major bleeding episodes were observed in 9 ECCO2R patients (36%). Transient thrombocytopenia probably related to the use of heparin has also been noticed [36, 43, 45]. However, thrombocytopenia as well as reduction in coagulation factors could also be the consequence of the interactions between the blood components and the circuit. In addition to the blood trauma caused by the pump and membrane, the contact between blood and the artificial surfaces of the circuit results in coagulation and fibrinolytic pathway activation and a complement-mediated inflammatory response [63]. Future research should be focused on improving anticoagulation protocols and developing practice guidelines [63, 64].
Concerns about mechanical events due to thrombosis persist. Despite anticoagulation protocols, the formation of clots in the circuits often occurs and contributes to reduced membrane CO2 clearance with a consequent rapid increase in PaCO2. The occurrence of membrane thrombosis has to be considered a life-threatening event and requires the prompt substitution of the circuit, changes in the ventilator settings, and endotracheal intubation in case of NIV [6, 36, 45]. To prevent clotting, particular attention should be paid to the choice of the vascular access and to detecting the kinking of catheters, as it can prevent the achievement of target blood flow rates [6]. Catheter displacement or kinking may cause pump malfunction and favors membrane clotting. Therefore, in cases of high body mass index and/or intraabdominal hypertension, subclavian or jugular vein catheterization may be preferred to the femoral veins, as it may better guarantee target blood flow rates without increasing the pressure in the circuit. Finally, episodes of intravascular hemolysis have been reported in two case series, with one requiring transfusion [6, 36].
Conclusion
ECCO2R may be a promising adjuvant therapeutic strategy for the management of patients with severe exacerbations of COPD and for the achievement of protective or ultra-protective ventilation in patients with ARDS without life-threatening hypoxemia. However, difficulties in predicting the progression of disease at an early stage of respiratory failure may limit the use of ECCO2R in clinical practice. Accordingly, careful clinical evaluation of the patients has to be performed to choose the most appropriate ECCO2R device in terms of extracorporeal blood flow rates. Furthermore, the potential complications from ECCO2R need to be considered, particularly the balance between bleeding and clotting events, and the optimal use of anticoagulation. Given the observational nature of most of the available clinical data and differences in technical features and performances of used devices, it is difficult to understand the balance of risks and benefits for or against ECCO2R in such patient populations. Therefore, ECCO2R should be considered an experimental technique rather than an accepted therapeutic strategy.
References
Brodie D, Bacchetta M (2011) Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 365:1905–1914
Del Sorbo L, Cypel M, Fan E (2014) Extracorporeal life support for adults with severe acute respiratory failure. Lancet Respir Med 2:154–164
Cove ME, MacLaren G, Federspiel WJ, Kellum JA (2012) Bench to bedside review: extracorporeal carbon dioxide removal, past present and future. Crit Care 16(5):232
Gattinoni L, Kolobow T, Damia G, Agostoni A, Pesenti A (1979) Extracorporeal carbon dioxide removal (ECCO2R): a new form of respiratory assistance. Int J Artif Organs 4:183–185
Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, Cancio LC (2011) Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med 39:1382–1387
Fanelli V, Ranieri MV, Mancebo J et al (2016) Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress syndrome. Crit Care 20:36
Del Sorbo L, Pisani L, Filippini C et al (2015) Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 43:120–127. doi:10.1097/CCM.0000000000000607
Gattinoni L, Agostoni A, Pesenti A et al (1980) Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet 2:292–294
Tajimi K, Kasai T, Nakatani T, Kobayashi K (1988) Extracorporeal lung assist for patient with hypercapnia due to status asthmaticus. Intensive Care Med 14:588–589
Sklar MC, Beloncle F, Katsios CM et al (2015) Extracorporeal carbon dioxide removal in patients with chronic obstructive pulmonary disease: a systematic review. Intensive Care Med 41:1752–1762
Schellongowski P, Riss K, Staudinger T et al (2015) Extracorporeal CO2 removal as bridge to lung transplantation in lifethreatening hypercapnia. Transpl Int 28:297–304
Tasker RC, Peters MJ (1998) Combined lung injury, meningitis and cerebral edema: how permissive can hypercapnia be? Intensive Care Med 24:616–619
Stengl M, Ledvinova L, Chvojka J et al (2013) Effects of clinically relevant acute hypercapnic and metabolic acidosis on the cardiovascular system: an experimental porcine study. Crit Care 17:R303
Ismaiel NM, Henzler D (2011) Effects of hypercapnia and hypercapnic acidosis on attenuation of ventilator-associated lung injury. Minerva Anestesiol 77:723–733
Nichol AD, O’Cronin DF, Howell K et al (2009) Infection-induced lung injury is worsened after renal buffering of hypercapnic acidosis. Crit Care Med 37:2953–2961
Karagiannidis C, Kampe KA, Sipmann FA et al (2014) Venovenous extracorporeal CO2 removal for the treatment of severe respiratory acidosis: pathophysiological and technical considerations. Critical Care 18(3):1
Morimont P, Batchinsky A, Lambermont B (2015) Update on the role of extracorporeal CO2 removal as an adjunct to mechanical ventilation in ARDS. Crit Care 19:117
Camporota L, Barrett N (2016) Current applications for the use of extracorporeal carbon dioxide removal in critically ill patients. Biomed Res Int 2016:9781695
Flörchinger B, Philipp A, Klose A et al (2008) Pumpless extracorporeal lung assist: a 10-year institutional experience. Ann Thorac Surg 86:410–417
Zanella A, Castagna L, Salerno D, Scaravilli V et al (2015) Respiratory electrodialysis. A novel, highly efficient extracorporeal CO2 removal technique. Am J Respir Crit Care Med 192:719–726
Scaravilli V, Kreyer S, Belenkiy S et al (2016) Extracorporeal carbon dioxide removal enhanced by lactic acid infusion in spontaneously breathing conscious sheep. Anesthesiology 124:674–682
Slutsky AS, Ranieri MV (2013) Ventilator-induced lung injury. N Engl J Med 22:2126–2136
Terragni PP, Rosboch G, Tealdi A et al (2007) Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 175:160–166
Terragni P, Ranieri VM, Brazzi L (2015) Novel approaches to minimize ventilator-induced lung injury. Curr Opin Crit Care 1:20–25
Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, Messa C, Pesenti A (2011) Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med 183:1193–1199
Grasso S, Stripoli T, De Michele M et al (2007) ARDSnet ventilator protocol and alveolar hyperinflation: role of positive end-expiratory pressure. Am J Respir Crit Care Med 176:761–767
Zapol WM, Snider MT, Hill JD et al (1979) Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 242:2193–2196
Kolobow T, Gattinoni L, Tomlinson TA, Pierce JE (1977) Control of breathing using an extracorporeal membrane lung. Anesthesiology 46:138–141
Gattinoni L, Agostoni A, Pesenti A et al (1980) Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet 2:292–294
Gattinoni L, Pesenti A, Mascheroni D et al (1986) Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA 256:881–886
Barthelemy R, Galletti PM, Trudell LA et al (1982) Total extracorporeal CO2 removal in a pumpless artery-to-vein shunt. Trans Am Soc Artif Intern Organs 28:354–358
Bein T, Weber F, Philipp A et al (2006) A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med 34:1372–1377. doi:10.1097/01.CCM.0000215111.85483.BD
Terragni PP, Rosboch G, Tealdi A et al (2007) Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 175:160–166
Terragni PP, del Sorbo L, Mascia L et al (2009) Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 111:826–835. doi:10.1097/ALN.0b013e3181b764d2
Bein T, Weber-Carstens S, Goldmann A et al (2013) Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus “conventional” protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 39:847–856. doi:10.1007/s00134-012-2787-6
Moss CE, Galtrey EJ, Camporota L, Meadows C, Gillon S, Ioannou N, Barrett NA (2016) A retrospective observational case series of low flow veno-venous extracorporeal carbon dioxide removal use in patients with respiratory failure. ASAIO J 62(4):458–462
Fitzgerald M, Millar J, Blackwood B, Davies A, Brett SJ, McAuley DF, McNamee JJ (2014) Extracorporeal carbon dioxide removal for patients with acute respiratory failure secondary to the acute respiratory distress syndrome: a systematic review. Crit Care 18:222
Grasso S, Stripoli T, Mazzone P et al (2014) Low respiratory rate plus minimally invasive extracorporeal CO2 removal decreases systemic and pulmonary inflammatory mediators in experimental acute respiratory distress syndrome. Crit Care Med 42:e451–e460. doi:10.1097/CCM.0000000000000312
Nava S, Hill N (2009) Non-invasive ventilation in acute respiratory failure. Lancet 374:250–259. doi:10.1016/S0140-6736(09)60496-7
Chandra D, Stamm JA, Taylor B et al (2012) Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med 185:152–159. doi:10.1164/rccm.201106-1094OC
Pisani L, Fasano L, Corcione N, Comellini V, Guerrieri A, Ranieri MV, Nava S (2015) Effects of extracorporeal CO2 removal on inspiratory effort and respiratory pattern in patients who fail weaning from mechanical ventilation. Am J Respir Crit Care Med 192:1392–1394. doi:10.1164/rccm.201505-0930LE
Kluge S, Braune SA, Engel M et al (2012) Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 38:1632–1639. doi:10.1007/s00134-012-2649-2
Burki NK, Mani RK, Herth FJF et al (2013) A novel extracorporeal CO2 removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 143:678–686. doi:10.1378/chest.12-0228
Braune S, Sieweke A, Brettner F et al (2016) The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med 42:1437–1444
Del Sorbo L, Fan E, Nava S, Ranieri VM (2016) ECCO2R in COPD exacerbation only for the right patients and with the right strategy. Intensive Care Med 42(11):1830–1831
Morelli A, D’Egidio A, Orecchioni A, Alessandri F, Mascia L, Ranieri M (2015) Extracorporeal CO2 removal in hypercapnic patients who fail noninvasive ventilation and refuse endotracheal intubation: a case series. Intensive Care Med Exp 3(Suppl 1):A824. doi:10.1186/2197-425X-3-S1-A824
Elliot SC, Paramasivam K, Oram J, Bodenham AR, Howell SJ, Mallick A (2007) Pumpless extracorporeal carbon dioxide removal for life-threatening asthma. Crit Care Med 35:945–948
Abrams DC, Brenner K, Burkart KM et al (2013) Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 10:307–314. doi:10.1513/AnnalsATS.201301-021OC
Roncon-Albuquerque R Jr, Carona G, Neves A, Miranda F, Castelo-Branco S, Oliveira T, Paiva JA (2014) Venovenous extracorporeal CO2 removal for early extubation in COPD exacerbations requiring invasive mechanical ventilation. Intensive Care Med 40:1969–1970
Hermann A, Staudinger T, Bojic A, Riss K, Wohlfarth P, Robak O, Sperr WR, Schellongowski P (2014) First experience with a new miniaturized pump-driven venovenous extracorporeal CO2 removal system (iLA Activve): a retrospective data analysis. ASAIO J 60:342–347
Schellongowski P, Riss K, Staudinger T et al (2015) Extracorporeal CO2 removal as bridge to lung transplantation in life threatening hypercapnia. Transplant Int 28:297–304
Collaud S, Benden C, Ganter C, et al. (2016) Extracorporeal life support as bridge to lung retransplantation: a multicenter pooled data analysis. Ann Thorac Surg 102(5):1680–1686
Redwan B, Ziegeler S, Semik M, Fichter J, Dickgreber N, Vieth V, Ernst EC, Fischer S (2016) Single-site cannulation veno-venous extracorporeal CO2 removal as bridge to lung volume reduction surgery in end-stage lung emphysema. ASAIO J 62(6):743–746
Aurigemma NM, Feldman NT, Gottlieb M, Ingram RH Jr, Lazarus JM, Lowrie EG (1977) Arterial oxygenation during hemodialysis. N Engl J Med 297:871–873
Gattinoni L, Kolobow T, Tomlinson T, Iapichino G, Samaja M, White D, Pierce J (1978) Low-frequency positive pressure ventilation with extracorporeal carbon dioxide removal (LFPPVECCO2R): an experimental study. Anesth Analg 57:470–477
Gattinoni L (2016) Ultra-protective ventilation and hypoxemia. Crit Care 20(1):130
Morimont P, Desaive T, Guiot J et al (2014) Effects of veno-venous CO2 removal therapy on pulmonary circulation in an ARDS model. Intensive Care Med Exp 2:45
Morimont P, Guiot J, T Desaive et al (2015) Veno-venous extracorporeal CO2 removal improves pulmonary hemodynamics in a porcine ARDS model. Acta Anaesthesiol Scand 59:448–456
Cherpanath TG, Landburg PP, Lagrand WK, Schultz MJ, Juffermans NP (2015) Effect of extracorporeal CO2 removal on right ventricular and hemodynamic parameters in a patient with acute respiratory distress syndrome. Perfusion 31(6):525–529
Karagiannidis C, Strassmann S, Philipp A, Müller T, Windisch W (2015) Veno-venous extracorporeal CO2 removal improves pulmonary hypertension in acute exacerbation of severe COPD. Intensive Care Med 41:1509–1510
Forster C, Schriewer J, John S, Eckardt KU, Willam C (2013) Low-flow CO2 removal integrated into a renal-replacement circuit can reduce acidosis and decrease vasopressor requirements. Crit Care 17:R154
Beloncle F, Brochard L (2015) Extracorporeal CO2 removal for chronic obstructive pulmonary disease: too risky or ready for a trial? Crit Care Med 43:245–246
Cardenas VJ Jr, Miller L, Lynch JE, Anderson MJ, Zwischenberger JB (2006) Percutaneous venovenous CO2 removal with regional anticoagulation in an ovine model. ASAIO J 52:467–470
Murphy DA, Hockings LE, Andrews RK et al (2015) Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 29(2):90–101
Hermann A, Riss K, Schellongowski P et al (2015) A novel pump-driven veno-venous gas exchange system during extracorporeal CO2 removal. Intensive Care Med 41:1773–1780
Fuehner T, Kuehn C, Hadem J et al (2012) Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 185:763–768
Acknowledgements
We would like to acknowledge Alberto Goffi, MD (Interdepartmental Division of Critical Care Medicine, University of Toronto, Toronto, Canada) for creating the figures for this manuscript. He was not compensated for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Antonio Pesenti received funding for research and travel from Maquet Cardiovascular, and is currently on the Medical Advisory Board of Novalung and Baxter. He holds a number of patents related to CO2 removal technology. All other authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Morelli, A., Del Sorbo, L., Pesenti, A. et al. Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med 43, 519–530 (2017). https://doi.org/10.1007/s00134-016-4673-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4673-0