Abstract
Hypoglycaemia (blood glucose concentration below the normal range) has been recognised as a complication of insulin treatment from the very first days of the discovery of insulin, and remains a major concern for people with diabetes, their families and healthcare professionals today. Acute hypoglycaemia stimulates a stress response that acts to restore circulating glucose, but plasma glucose concentrations can still fall too low to sustain normal brain function and cardiac rhythm. There are long-term consequences of recurrent hypoglycaemia, which are still not fully understood. This paper reviews our current understanding of the acute and cumulative consequences of hypoglycaemia in insulin-treated diabetes.
Graphical abstract

Similar content being viewed by others


Introduction
Hypoglycaemia as an adverse consequence of diabetes therapies has been known since the early days of insulin’s discovery. In their book, ‘Breakthrough: Elizabeth Hughes, the discovery of insulin, and the making of a medical miracle’, Thea Cooper and Arthur Ainsberg describe how Elizabeth Hughes, diagnosed with type 1 diabetes in 1919, and her nurse, Blanche ‘shared a bed in the hope that Blanche could better detect and response to a night time attack’ and that Blanche saved Elizabeth’s life ‘dozens of times … with orange juice or a molasses kiss’ [1]. Describing events in 1922, the authors note that ‘several diabetics had suffered hypoglycaemic shock’ [1]. In purifying insulin, James Collip defined a unit of it as the amount that would send a rabbit into hypoglycaemic seizure. One hundred years later, hypoglycaemia remains a risk for everyone taking insulin [1].
Definitions of hypoglycaemia
Hypoglycaemia is defined as a concentration of glucose in the blood that is lower than normal, traditionally defined biochemically as a plasma glucose of <3.5 mmol/l. A precise biochemical definition is complex, however. Blood glucose concentration is a continuum and the value that is associated with harm depends on the harm that is being described. Homeostatic responses begin as soon as plasma glucose starts to fall, with cessation of endogenous insulin secretion and stimulation of pancreatic glucagon noted at plasma glucose values of around 4 mmol/l. Evidence for material harm, such as impaired cognition, cardiac arrythmia, defective responses to subsequent low glucose values and associations with mortality rate has accrued for a plasma glucose value of less than 3 mmol/l [2]. Such evidence has underpinned a consensus statement from the International Hypoglycaemia Study Group, endorsed by the American Diabetes Association, the European Association for the Study of Diabetes and some charitable and patient groups, which defines three levels of plasma glucose that are important to use when describing low-glucose episodes caused by diabetes therapies (Table 1) [2, 3].
For people with diabetes, hypoglycaemia may be better defined by the clinical picture and by the degree of distress and disruption an episode may cause, ranging from having to ingest carbohydrate when not wishing for it, to unpleasant but protective symptoms of an acute stress response, to confusion and coma. The American Diabetes Association categorises hypoglycaemic episodes into: severe (requiring third party intervention because of cognitive impairment too great to support self-treatment); documented symptomatic (also now referred to as non-severe and described as symptomatic episodes confirmed by a low blood glucose measurement and by definition, self-treated); asymptomatic (detected by testing only); probable symptomatic (symptoms of hypoglycaemia but not confirmed by a measurement); and pseudohypoglycaemia (symptoms of hypoglycaemia accompanied by a non-hypoglycaemic blood glucose concentration) [4].
The acute consequences of hypoglycaemia
Symptomatic stress response and impaired response to subsequent hypoglycaemic episodes
Hypoglycaemia elicits a stress response, which acts to correct the glucose fall. In health, cessation of insulin secretion and stimulation of pancreatic glucagon, driven by both local and central neuroendocrine signalling, abort a plasma glucose fall through stimulation of hepatic endogenous glucose production. In insulin-deficient diabetes, where exogenous insulin or an insulin secretagogue is being taken, circulating insulin levels remain elevated and pancreatic alpha cells, unable to detect a signal from falling beta cell stimulation, do not secrete glucagon [5, 6]. Correction of the falling glucose is, therefore, dependent on hyperglycaemic sympathetic nerve stimulation, catecholamine secretion and, critically, the person recognising the hypoglycaemia and ingesting carbohydrate. The plasma glucose concentration at which these responses are triggered is influenced by prior glycaemic experience: people with poorly controlled type 2 diabetes and no previous experience of hypoglycaemia may experience some elements of the stress response, and certainly symptoms, at higher plasma glucose concentration than occurs in health [7], and people with previous experience of hypoglycaemia may downregulate the glucose concentration at which sympathetic and hormonal responses to a falling glucose occur, sometimes to a value below that at which cognitive deterioration starts [8, 9]. This creates a syndrome of impaired awareness of hypoglycaemia, in which failure of subjective awareness of hypoglycaemia increases risk of severe hypoglycaemia sixfold in people with type 1 diabetes (in whom severe hypoglycaemia is more common) and 17-fold in individuals with type 2 diabetes who are taking insulin [10, 11]. Parenthetically, we should note that other factors can affect the hierarchy of responses to hypoglycaemia. For example, age can have an impact on response to hypoglycaemia, with findings showing that older people have a lower glucose concentration for subjective awareness and hormonal responses, while the deterioration of cognitive function is preserved in these individuals at an arterialised plasma glucose of 3 mmol/l [12]. Furthermore, in children and in elderly people with diabetes, cognitive and behavioural signs and symptoms may be more prominent in the clinical presentation [13, 14].
We have thus described the first two important acute consequences of hypoglycaemia: the symptomatic stress response and the impairment of responses to subsequent episodes. In addition, we have implied a third: impairment of cognitive function during an acute episode.
Neurological consequences
The impairment of cognitive function associated with hypoglycaemia ranges from slowing of cerebration and performance, confusion, irrational behaviour and drowsiness, to coma and seizures. Other outcomes include inconvenience, embarrassment, and physical injury to the patient or to others, with possible employment, social and legal implications. There is evidence that the threshold for cognitive impairment changes less in response to antecedent hypoglycaemia than does the threshold for subjective awareness and neurohumoral responses, which provides a potential mechanism for the increased risk of severe hypoglycaemia, as the person becomes unable to self-treat [15, 16].
Other neurological consequences of a hypoglycaemic episode include temporary focal deficits, including Todd’s paresis, in which the person wakes after an undetected nocturnal hypoglycaemia with symptoms and signs mimicking a stroke [17]. Complete recovery occurs within hours and there is no prognostic implication to these episodes. We will discuss below whether residual cognitive deficit results from hypoglycaemia from which an apparently complete recovery is made at the time. Here we note only that in animal studies of extreme hypoglycaemia, the hippocampus seems particularly vulnerable. Anecdotally, memory deficits are reported by people with type 1 diabetes and problematic hypoglycaemia, with some evidence to support an association [18], but hypoglycaemia impairs formation and consolidation of memory in type 1 diabetes acutely [19, 20] and whether the memory deficits are resolved by hypoglycaemia avoidance remains to be determined.
Hypoglycaemia is associated with changes in regional brain activation, not just in the region of the hypothalamic–pituitary axis, but also in brain regions involved in interoception (relevant to symptom generation and perception) and in regions involved in emotional salience, aversion, executive function and memory [21]. Long duration type 1 diabetes can alter these regional brain responses and it has been postulated that enhanced thalamic activity in hypoglycaemia in type 1 diabetes may support subjective awareness in the face of reduced catecholamine responses [22].
Cardiovascular consequences
Other potential acute consequences of hypoglycaemic episodes include cardiovascular effects, both as a result of the stress response (tachycardia, widened arterial pulse pressure) and arrythmias [23, 24], including lengthening of the QT interval (QTc) on an electrocardiogram and bradycardia. Such arrythmias are associated with the adrenergic responses and the fall in plasma potassium associated with hypoglycaemia [25]. Hypoglycaemia produces endothelial dysfunction (as does hyperglycaemia) [26], an inflammatory response [27], and creates a coagulopathy, which can last for several days [28].
Mortality
Death during an acute hypoglycaemic episode is rare but does occur, accounting for up to 10% of deaths in people with type 1 diabetes under the age of 40 years, in whom other causes of death are, happily, rare [29].
Nocturnal hypoglycaemia
Over 50% of severe hypoglycaemic episodes in insulin-treated diabetes occur at night and this topic has been reviewed previously [30]. Hypoglycaemia at night has been shown to impact on recognition of hypoglycaemia the next day [31] and, as already discussed, may have an impact on the consolidation of memory that should happen during sleep [20]. The counterregulatory responses to hypoglycaemia are suppressed during deep sleep, so episodes may remain asymptomatic and undetected [32]. There is little evidence for an impact on cognitive function the next day, but mood and well-being have been described as adversely affected [30]. Rare, unexpected nocturnal deaths have been reported in young people with type 1 diabetes, attributed to hypoglycaemia and accounting for 5% of all deaths in this population [33]. The physiological and psychological impact of asymptomatic nocturnal hypoglycaemia that is recognised on waking by people using retrospective intermittently monitored glucose meters vs hypoglycaemia detected at the time by alarms from real-time continuous glucose monitoring remains to be determined.
The evolution of acute responses to hypoglycaemia over time
The main driver of hypoglycaemia in diabetes is glucose lowering therapy: exogenous insulin or insulin secretagogues, such as sulfonylureas. Their effects are exacerbated by the defects in the counterregulatory responses to a falling blood glucose that occur in insulin-deficient diabetes (described above), including the loss of glucagon responses and, later, impaired sympathetic and catecholamine responses [5]. Severe hypoglycaemia occurs at a rate of 1.3 episodes per person per year in people with type 1 diabetes; but its occurrence is extremely skewed, with only 40% of individuals experiencing an episode in any one year, and 10% of adults with type 1 diabetes contributing nearly 70% of all episodes in the year, with many of these people reporting recurrent events [34]. Interestingly, a recent study of people with insulin-treated type 2 diabetes in the Netherlands described only slightly lower prevalence of severe hypoglycaemia (32%) [35]. Impaired awareness of hypoglycaemia is a major risk factor in both types of diabetes.
The cumulative impact of hypoglycaemia
The common outcome of any hypoglycaemic episode is complete recovery, but we have already begun to allude to possible impacts of hypoglycaemia on future health.
Psychological, societal and economic impact
There is a psychological impact from hypoglycaemia experiences. Any episode can be unpleasant: non-severe episodes can be associated with unpleasant symptoms, which may be distressing to the person experiencing them to the extent that the individual will describe the episode as ‘very severe’. One person with diabetes described multiple negative experiences of hypoglycaemia including interruption of activities, sleep disruption and a ‘horrible force feeding one has to endure if not remotely hungry’ (personal communication with author). Fear of hypoglycaemia may develop and lead to behaviours that are unhelpful either to diabetes control or to normal social interaction [36]. One recent study described 40–50% of a clinic population of adults with type 1 diabetes as expressing fear of hypoglycaemia [37] and, in people with type 2 diabetes, non-severe hypoglycaemia may have as big an impact on fear of hypoglycaemia as severe episodes, with negative impact on quality of life and on glucose management strategies [38, 39]. Other family members, perhaps particularly parents of children with diabetes, are also affected by fear of hypoglycaemia [40], with partners of people with problematic hypoglycaemia (impaired awareness of hypoglycaemia and recurrent severe episodes) expressing very high distress [41]. The societal impact may include loss of driving privileges, restricted employment, and breakdown of family relationships, with restricted access to children. There are potential economic impacts beyond the costs to healthcare providers (who are involved in a tiny minority of cases), including time lost from work, that have still to be fully understood [42].
Impaired awareness of hypoglycaemia
Impaired awareness of hypoglycaemia is believed to arise as a result of recurrent exposure to hypoglycaemic episodes (under 3 mmol/l), with evidence that avoidance of such exposure can restore awareness, both in mechanistic studies (e.g. [43]), and clinically [44]. McCrimmon’s group has described this as a conditioning response [45]. A small but highly vulnerable group of patients seem resistant to interventions for hypoglycaemia avoidance [46]. Some may have unhelpful cognitions around hypoglycaemia, which may act as barriers to future hypoglycaemia avoidance [47, 48]. One clinic-based study of people with type 1 diabetes found that one-third of people at high risk for severe hypoglycaemia expressed low concern about their hypoglycaemia [49]. Neuroimaging studies offer a potential explanation, with altered activation of brain regions involved in appetite control, emotional salience, aversion, recall, arousal and decision making in response to hypoglycaemia in people with established impaired awareness and recurrent severe hypoglycaemia [50, 51]. Interventions that target cognitions around hypoglycaemia may be helpful in this group [52].
Risk of future mortality
There is a growing body of evidence linking severe hypoglycaemia to future mortality both in hospital and in the community, with estimates of increased risk ranging from 50% to 600% [53]. Some of the responses to acute hypoglycaemia, particularly the proinflammatory and coagulopathy effects, have been implicated as underlying mechanisms, but the data remain compatible with hypoglycaemia being a marker of frailty and high risk of death [53]. Severe hypoglycaemia has been associated with approximately a doubling in risk of subsequent cardiovascular events, including death, but the relationship is bi-directional [54].
Cognitive function
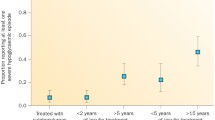
Meanwhile, concern remains that recurrent hypoglycaemia may have a lasting impact on cognitive function. There is some evidence that recurrent severe hypoglycaemia in children at an age when the brain is still developing may result in subtle impairment of performance on cognitive function testing in adolescence, especially when seizures have accompanied the hypoglycaemia [55, 56]. In adults data remain controversial, with consistently reassuring data coming from long-term follow-up of the DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) cohort of type 1 diabetes patients and from the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial in people with type 2 diabetes [57, 58]. However, a recent large study in older adults with type 1 diabetes demonstrated an association between patient-reported history of severe hypoglycaemia events (episodes resulting in emergency department or inpatient admission), either recent (any) or life-time exposure, and impaired performance in global cognition, language and executive function and recent episodic memory, with evidence for a dose effect [59]. An earlier meta-analysis also found a bi-directional relationship between dementia and hypoglycaemia in older adults [60].
Conclusions
Hypoglycaemia has been a complication of diabetes treatments since the discovery of insulin, and remains a major concern for people with diabetes, their families and friends, and healthcare professionals and providers. Experiencing hypoglycaemia has consequences, as summarised in Fig. 1. Individual episodes of hypoglycaemia are inconvenient, unpleasant and potentially dangerous and recurrent episodes have long-term negative health implications that are still being explored [61]. Minimising hypoglycaemic exposure must remain a key target for diabetes therapies and hypoglycaemia experience must always be a metric for assessing the effectiveness of diabetes management.
A graphical summary of the potential consequences of hypoglycaemia. Elements of the stress response to a hypoglycaemic episode are shown in orange text boxes; other colours indicate different classes of possible consequences of hypoglycaemic episodes and of hypoglycaemia itself (blue, neurological/cognitive; purple, psychological; yellow, socioeconomic; brown, mortality; green, cardiovascular). This figure is available as a downloadable slide
References
Cooper T, Ainsberg A (2011) Breakthrough: Elizabeth Hughes, the Discovery of Insulin, and the Making of a Medical Miracle. St Martin’s Press, New York City
The International Hypoglycaemia Study Group (2017) Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 60:3–6. https://doi.org/10.1007/s00125-016-4146-6
Battelino T, Danne T, Bergenstal RM et al (2019) Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 42:1593–1603. https://doi.org/10.2337/dci19-0028
Seaquist ER, Anderson J, Childs B et al (2013) Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 36:1384–1395. https://doi.org/10.2337/dc12-2480
Bolli G, de Feo P, Compagnucci P et al (1983) Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 32:134–141. https://doi.org/10.2337/diab.32.2.134
Peacey SR, Rostami-Hodjegan A, George E, Tucker GT, Heller SR (1997) The use of tolbutamide-induced hypoglycemia to examine the intraislet role of insulin in mediating glucagon release in normal humans. J Clin Endocrinol Metab 82:1458–1461
Spyer G, Hattersley AT, MacDonald IA, Amiel S, MacLeod KM (2000) Hypoglycaemic counter-regulation at normal blood glucose concentrations in patients with well controlled type-2 diabetes. Lancet 356(9246):1970–1974
Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV (1988) Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 37:901–907. https://doi.org/10.2337/diab.37.7.901
Heller SR, Cryer PE (1991) Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 40:223–226. https://doi.org/10.2337/diab.40.2.223
Geddes J, Schopman JE, Zammitt NN, Frier BM (2008) Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med 25:501–504. https://doi.org/10.1111/j.1464-5491.2008.02413.x
Schopman JE, Geddes J, Frier BM (2010) Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract 87:64–68. https://doi.org/10.1016/j.diabres.2009.10.013
Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA (1997) Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care 20:135–141. https://doi.org/10.2337/diacare.20.2.135
Graveling AJ, Noyes KJ, Allerhand MH et al (2014) Prevalence of impaired awareness of hypoglycemia and identification of predictive symptoms in children and adolescents with type 1 diabetes. Pediatr Diabetes 15:206–213. https://doi.org/10.1111/pedi.12077
Jaap AJ, Jones GC, McCrimmon RJ, Deary IJ, Frier BM (1998) Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet Med 15:398–401. https://doi.org/10.1002/(SICI)1096-9136(199805)15:5<398::AID-DIA595>3.0.CO;2-B
Maran A, Lomas J, Macdonald IA, Amiel SA (1995) Lack of preservation of higher brain function during hypoglycaemia in patients with intensively-treated IDDM. Diabetologia 38:1412–1418. https://doi.org/10.1007/BF00400601
Hvidberg A, Fanelli CG, Hershey T, Terkamp C, Craft S, Cryer PE (1996) Impact of recent antecedent hypoglycemia on hypoglycemic cognitive dysfunction in nondiabetic humans. Diabetes 45:1030–1036. https://doi.org/10.2337/diab.45.8.1030
Fernandes PM, Whiteley WN, Hart SR, Al-Shahi Salman R (2013) Strokes: mimics and chameleons. Pract Neurol 13:21–28. https://doi.org/10.1136/practneurol-2012-000465
Chen YX, Liu ZR, Yu Y, Yao ES, Liu XH, Liu L (2017) Effect of recurrent severe hypoglycemia on cognitive performance in adult patients with diabetes: A meta-analysis. J Huazhong Univ Sci Technolog Med Sci 37:642–648. https://doi.org/10.1007/s11596-017-1784-y
Allen KV, Pickering MJ, Zammitt NN et al (2015) Effects of acute hypoglycemia on working memory and language processing in adults with and without type 1 diabetes. Diabetes Care 38:1108–1115. https://doi.org/10.2337/dc14-1657
Jauch-Chara K, Hallschmid M, Gais S et al (2007) Hypoglycemia during sleep impairs consolidation of declarative memory in type 1 diabetic and healthy humans. Diabetes Care 30:2040–2045. https://doi.org/10.2337/dc07-0067
Teh MM, Dunn JT, Choudhary P et al (2010) Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. Neuroimage 53:584–592. https://doi.org/10.1016/j.neuroimage.2010.06.033
Nwokolo M, Amiel SA, O’Daly O et al (2020) Hypoglycemic thalamic activation in type 1 diabetes is associated with preserved symptoms despite reduced epinephrine. J Cereb Blood Flow Metab 40:787–798. https://doi.org/10.1177/0271678X19842680
Novodvorsky P, Bernjak A, Chow E et al (2017) Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Diabetes Care 40:655–662. https://doi.org/10.2337/dc16-2177
Chow E, Bernjak A, Williams S et al (2014) Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 63:1738–1747. https://doi.org/10.2337/db13-0468
Lee S, Harris ND, Robinson RT, Yeoh L, Macdonald IA, Heller SR (2003) Effects of adrenaline and potassium on QTc interval and QT dispersion in man. Eur J Clin Investig 3:93–98
Joy NG, Perkins JM, Mikeladze M, Younk L, Tate DB, Davis SN (2016) Comparative effects of acute hypoglycemia and hyperglycemia on pro-atherothrombotic biomarkers and endothelial function in non-diabetic humans. J Diabetes Complicat 30:1275–1281. https://doi.org/10.1016/j.jdiacomp.2016.06.030
Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN (2010) Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 33:1529–1535. https://doi.org/10.2337/dc09-0354
Chow E, Iqbal A, Walkinshaw E et al (2018) Prolonged Prothrombotic Effects of Antecedent Hypoglycemia in Individuals With Type 2 Diabetes. Diabetes Care 41:2625–2633. https://doi.org/10.2337/dc18-0050
Cryer PE (2012) Severe hypoglycemia predicts mortality in diabetes. Diabetes Care 35:1814–1816. https://doi.org/10.2337/dc12-0749
Graveling AJ, Frier BM (2017) The risks of nocturnal hypoglycaemia in insulin-treated diabetes. Diabetes Res Clin Pract 133:30–39. https://doi.org/10.1016/j.diabres.2017.08.012
Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J (1993) Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes 42(9):1233–1237. https://doi.org/10.2337/diab.42.9.1233
Jones TW, Porter P, Sherwin RS et al (1998) Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 338:1657–1662. https://doi.org/10.1056/NEJM199806043382303
Gagnum V, Stene LC, Jenssen TG et al (2017) Causes of death in childhood-onset type 1 diabetes: long-term follow-up. Diabet Med 34:56–63. https://doi.org/10.1111/dme.13114
Pedersen-Bjergaard U, Pramming S, Heller SR et al (2004) Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev 20:479–486. https://doi.org/10.1002/dmrr.482
van Meijel LA, de Vegt F, Abbink EJ et al (2020) High prevalence of impaired awareness of hypoglycemia and severe hypoglycemia among people with insulin-treated type 2 diabetes: The Dutch Diabetes Pearl Cohort. BMJ Open Diabetes Res Care 8:e000935. https://doi.org/10.1136/bmjdrc-2019-000935
Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L (2007) A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns 68(1):10–15. https://doi.org/10.1016/j.pec.2007.05.003
Castellano-Guerrero AM, Guerrero R, Relimpio F et al (2018) Prevalence and predictors of depression and anxiety in adult patients with type 1 diabetes in tertiary care setting. Acta Diabetol 55:943–953. https://doi.org/10.1007/s00592-018-1172-5
Rossi MC, Nicolucci A, Ozzello A et al (2019) HYPOS-1 Study Group of AMD. Impact of severe and symptomatic hypoglycemia on quality of life and fear of hypoglycemia in type 1 and type 2 diabetes. Results of the Hypos-1 observational study. Nutr Metab Cardiovasc Dis 29:736–743. https://doi.org/10.1016/j.numecd.2019.04.009
Hendrieckx C, Ivory N, Singh H, Frier BM, Speight J (2019) Impact of severe hypoglycaemia on psychological outcomes in adults with Type 2 diabetes: a systematic review. Diabet Med 36(9):1082–1091. https://doi.org/10.1111/dme.14067
Pate T, Klemenčič S, Battelino T, Bratina N (2019) Fear of hypoglycemia, anxiety, and subjective well-being in parents of children and adolescents with type 1 diabetes. J Health Psychol 24:209–218. https://doi.org/10.1177/1359105316650931
Lawton J, Rankin D, Elliott J et al (2014) Experiences, views, and support needs of family members of people with hypoglycemia unawareness: interview study. Diabetes Care 37:109–115
Aronson R, Galstyan G, Goldfracht M, Al Sifri S, Elliott L, Khunti K (2018) Direct and indirect health economic impact of hypoglycaemia in a global population of patients with insulin-treated diabetes. Diabetes Res Clin Pract 138:35–43. https://doi.org/10.1016/j.diabres.2018.01.007
Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA (1994) Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 344(8918):283–287. https://doi.org/10.1016/S0140-6736(94)91336-6
Yeoh E, Choudhary P, Nwokolo M, Ayis S, Amiel SA (2015) Interventions That Restore Awareness of Hypoglycemia in Adults With Type 1 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 38:1592–1609. https://doi.org/10.2337/dc15-0102
Farrell CM, McNeilly AD, Fournier P et al (2020) A randomised controlled study of high intensity exercise as a dishabituating stimulus to improve hypoglycaemia awareness in people with type 1 diabetes: a proof-of-concept study. Diabetologia 63:853–863. https://doi.org/10.1007/s00125-019-05076-5
Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA (2009) Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Diabetes Care 32:1196–1198
Rogers HA, de Zoysa N, Amiel SA (2012) Patient experience of hypoglycaemia unawareness in Type 1 diabetes: are patients appropriately concerned? Diabet Med 29:321–327. https://doi.org/10.1111/j.1464-5491.2011.03444.x
Cook AJ, DuBose SN, Foster N et al (2019) Cognitions Associated With Hypoglycemia Awareness Status and Severe Hypoglycemia Experience in Adults With Type 1 Diabetes. Diabetes Care 42:1854–1864. https://doi.org/10.2337/dc19-0002
Anderbro TC, Amsberg S, Moberg E et al (2018) A longitudinal study of fear of hypoglycaemia in adults with type 1 diabetes. Endocrinol Diabetes Metab 1:e00013. https://doi.org/10.1002/edm2.13
Dunn JT, Choudhary P, Teh MM et al (2018) The impact of hypoglycaemia awareness status on regional brain responses to acute hypoglycaemia in men with type 1 diabetes. Diabetologia 61:1676–1687. https://doi.org/10.1007/s00125-018-4622-2
Nwokolo M, Amiel SA, O’Daly O et al (2019) Impaired Awareness of Hypoglycemia Disrupts Blood Flow to Brain Regions Involved in Arousal and Decision Making in Type 1 Diabetes. Diabetes Care 42:2127–2135. https://doi.org/10.2337/dc19-0337
de Zoysa N, Rogers H, Stadler M et al (2014) A psychoeducational program to restore hypoglycemia awareness: the DAFNE-HART pilot study. Diabetes Care 37:863–866
The International Hypoglycaemia Study Group (2019) Hypoglycaemia, cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol 7:385–396. https://doi.org/10.1016/S2213-8587(18)30315-2
Standl E, Stevens SR, Lokhnygina Y et al (2020) Confirming the Bidirectional Nature of the Association Between Severe Hypoglycemic and Cardiovascular Events in Type 2 Diabetes: Insights From EXSCEL. Diabetes Care 43:1–10
Blasetti A, Chiuri RM, Tocco AM et al (2011) The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol 26:1383–1391. https://doi.org/10.1177/0883073811406730
He J, Ryder AG, Li S, Liu W, Zhu X (2018) Glycemic extremes are related to cognitive dysfunction in children with type 1 diabetes: A meta-analysis. J Diabetes Investig 9:1342–1353. https://doi.org/10.1111/jdi.12840
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group (2007) Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356(18):1842–1852. https://doi.org/10.1056/NEJMoa066397
Cukierman-Yaffe T, Bosch J, Jung H, Punthakee Z, Gerstein HC (2019) Hypoglycemia and Incident Cognitive Dysfunction: A Post Hoc Analysis From the ORIGIN Trial. Diabetes Care 42:142–147
Lacy ME, Gilsanz P, Eng C, Beeri MS, Karter AJ, Whitmer RA (2020) Severe Hypoglycemia and Cognitive Function in Older Adults With Type 1 Diabetes: The Study of Longevity in Diabetes (SOLID). Diabetes Care 43:541–548. https://doi.org/10.2337/dc19-0906
Mattishent K, Loke YK (2016) Bi-directional interaction between hypoglycaemia and cognitive impairment in elderly patients treated with glucose-lowering agents: a systematic review and meta-analysis. Diabetes Obes Metab 18:135–141. https://doi.org/10.1111/dom.12587
de Galan BE, McCrimmon RJ, Ibberson M et al (2020) Hypo-RESOLVE consortium. Reducing the burden of hypoglycaemia in people with diabetes through increased understanding: design of the Hypoglycaemia REdefining SOLutions for better liVEs (Hypo-RESOLVE) project. Diabet Med 37:1066–1073. https://doi.org/10.1111/dme.14240
Acknowledgements
My thanks to L. Warren, M. Kendall and the members of the HARPdoc user group (King’s College London, London, UK) for discussions about hypoglycaemia from the perspective of the people with diabetes. Also to my research colleagues in the Diabetes Research Group and Centre for Neuroimaging Sciences, both at King’s College London (London, UK), and my clinical colleagues and patients at King’s College Hospital NHS Foundation Trust, without whom much of the work reported here would not have occurred.
Author’s relationships and activities
SAA has served on advisory boards for NovoNordisk, Abbott UK and Roche and spoken at a meeting sponsored by Sanofi in the last 36 months.
Author information
Authors and Affiliations
Contributions
This review is solely the work of the author.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Figure slide
(PPTX 209 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amiel, S.A. The consequences of hypoglycaemia. Diabetologia 64, 963–970 (2021). https://doi.org/10.1007/s00125-020-05366-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05366-3