Abstract
Aims/hypothesis
The aim of this work was to investigate whether tetraspanin 7 autoantibodies (TSPAN7A) are valuable in predicting poor beta cell function in individuals with latent autoimmune diabetes in adults (LADA).
Methods
The cross-sectional study involved participants with LADA (n = 173), type 1 diabetes (n = 158), type 2 diabetes (n = 204) and healthy control participants (n = 170). The longitudinal study involved 53 participants with LADA, with a 3-year follow-up. In both cohorts, TSPAN7A in the sera were measured by a luciferase immunoprecipitation system assay, and physical and clinical characteristics were recorded.
Results
The prevalence of TSPAN7A in LADA, type 1 diabetes, type 2 diabetes and healthy control participants was 21.4% (37/173), 26% (41/158), 0.5% (1/204) and 1.2% (2/170), respectively. Importantly, measurement of TSPAN7A significantly increased the number of individuals with LADA found to be positive for multiple antibodies (32.4% vs 22%; p < 0.001). Further logistic regression analysis demonstrated that positivity for TSPAN7A (OR 2.87, p = 0.034), disease duration (OR 1.83, p = 0.019) and GAD antibody titre (OR 2.67, p = 0.009) were risk factors for beta cell function in LADA, while BMI (OR 0.34, p = 0.001) was a protective factor. In the prospective study in individuals with LADA, the median annual decrease in rates of fasting C-peptide and 2 h postprandial C-peptide in individuals who were positive for TSPAN7A was significantly higher when compared with the decrease in those who were negative for TSPAN7A (34.6% vs 7.9%, p = 0.043 and 33.2% vs 11%, p = 0.041, respectively).
Conclusions/interpretation
TSPAN7A are valid islet autoantibodies for use in East Asian populations with autoimmune diabetes and can discriminate individuals with LADA who have lower beta cell function after disease progression.
Similar content being viewed by others

Introduction
Latent autoimmune diabetes in adults (LADA) is type 1 diabetes with an onset in adulthood and a slow progression. In LADA, beta cell failure occurs slowly and insulin treatment is not required within at least 6 months of diagnosis [1,2,3]. Islet autoantibodies are key markers of islet autoimmunity in LADA and type 1 diabetes [4]. Five major autoantibody types have been identified in autoimmune diabetes—islet cell autoantibodies (ICA), GAD autoantibodies (GADA), insulin autoantibodies (IAA), protein tyrosine phosphatase autoantibodies (IA-2A) and zinc transporter 8 autoantibodies (ZnT8A). A sixth type, tetraspanin 7 autoantibodies (TSPAN7A), has been identified only recently [5, 6].
Tetraspanin 7 (TSPAN7) is a member of the transmembrane 4 superfamily, also called the tetraspanin family [7]. Tetraspanin family proteins are characterised by four transmembrane domains, with one short and one large extracellular loop. TSPAN7 is a glycoprotein located at the cell surface and is expressed in the brain, lung and pancreas [5]. In the pancreas, TSPAN7 is specifically expressed in the islets [8]. Previous studies have found that TSPAN7A are present in 19–43% of white individuals with type 1 diabetes [5, 6, 9, 10]. Unfortunately, its diagnostic value for type 1 diabetes is limited. However, the prevalence and clinical value of TSPAN7A in individuals with LADA have not been reported; in particular, information regarding their association with beta cell function is lacking. Therefore, the major aim of our study was to evaluate the prevalence of TSPAN7A and their potential clinical value in individuals with LADA. Furthermore, we investigated the frequency of TSPAN7A in Chinese individuals with type 1 diabetes because all previous studies have been restricted to white people.
Methods
Study participants
Individuals with LADA (n = 173), type 2 diabetes (n = 204) and type 1 diabetes (n = 158) and healthy control individuals (n = 170) were enrolled from the Second Xiangya Hospital of Central South University (Changsha, Hunan, China) from May 2014 to August 2017 for the cross-sectional study. Another 157 individuals with type 1 diabetes (duration >1 year) were recruited to examine the change in the time course of TSPAN7A, GADA, ZnT8A and IA-2A prevalence. Another 53 individuals with LADA were enrolled from the Second Xiangya Hospital of Central South University from October 2010 to December 2013 for a follow-up study in which clinical characteristics were determined annually for 3 years. Sera from another 199 healthy participants were collected from the Second Xiangya Hospital of Central South University to establish the threshold of positivity for TSPAN7A. The criteria of the WHO (version 1999) were applied to the diagnosis of diabetes [11]: fasting plasma glucose (FPG) ≥7 mmol/l or 2 h plasma glucose during 75 g OGTT ≥11.1 mmol/l with at least one obvious diabetic symptom (thirst, polyuria or unexplained weight loss). For asymptomatic individuals, a confirmatory test was conducted on another day. For children, the oral glucose load was based on body weight (1.75 g/kg). Individuals with LADA were recruited based on the criteria used in previous studies [2, 12]: (1) positive for at least one autoantibody (GADA, IA-2A or ZnT8A); (2) age ≥30 years at onset of diabetes and (3) at least 6 months of therapy without insulin. Type 1 diabetes was diagnosed according to the following criteria: acute-onset ketosis or ketoacidosis; reliance on insulin therapy; impaired islet function or islet autoantibody (GADA, IA-2A or ZnT8A) positivity. The individuals with type 1 diabetes presented with diabetes within 1 year. All individuals with type 2 diabetes were negative for GADA. All healthy participants underwent a standard 75 g OGTT to confirm that their blood glucose was normal. The exclusion criteria for healthy participants were: (1) other autoimmune diseases; (2) infectious disease; (3) pregnancy; (4) history of immunosuppressive drugs or steroids >7 days and (5) malignant disease. This study was approved by the ethics committee of the Second Xiangya Hospital of Central South University and all participants or their parents gave written informed consent.
Physical characteristics (sex, age, body height and weight) were recorded by professional researchers. Fasting blood was used for measurement of FPG, HbA1c and fasting C-peptide (FCP). Postprandial blood samples were used to test 2 h postprandial plasma glucose (PPG) and 2 h postprandial C-peptide (PCP).
C-peptide and HbA1c assays
FCP and PCP were measured by a chemiluminescence method using the Adiva Centaur Systema kit (Siemens, Munich, Germany). HbA1c was detected by automated liquid chromatography (VARIANT II Hemoglobin Testing System; Bio-Rad Laboratories, Hercules, CA, USA).
Islet autoantibody assays
Islet autoantibodies, including GADA, IA-2A and ZnT8A, were detected by radioligand assays in duplicate [13]. Based on the 99th percentile observed in 405 healthy participants, the cut-off values of positivity for GADA and IA-2A were 18 U/ml and 3.3 U/ml of WHO units, respectively. The threshold of antibody index for ZnT8A was 0.011 [14]. For antibody-positive individuals, blood was drawn again within 7 days to exclude false-positive results. In the 2016 islet autoantibody standardization programme (IASP 2016), the sensitivity and specificity in our laboratory were, respectively, 82% and 97.8% for GADA, 76% and 100% for IA-2A and 72% and 100% for ZnT8A.
Cloning and expression of TSPAN7
Luciferase-tagged full-length TSPAN7 was cloned into the pREN-2 vector, which was kindly provided by P. D. Burbelo (National Institute of Dental and Craniofacial Research, National Institutes of Health, MD, USA) [15]. The untagged TSPAN7 expression vector was purchased from Origene (Rockville, MD, USA). Plasmids with full-length TSPAN7 or untagged TSPAN7 were transfected into 293T cells cultured in DMEM supplemented with 10% FBS and 100 U/ml penicillin–streptomycin (Gibco, Carlsbad, CA, USA). After 48 h, cells were lysed in Glo Lysis Buffer (Promega, Madison, WI, USA) for 15 min at 4°C followed by centrifugation (12,000 g at 4°C for 15 min). The supernatant fraction was collected for further testing.
Luciferase immunoprecipitation system assay for TSPAN7A
TSPAN7A were assayed in duplicate by a luciferase immunoprecipitation assay (LIPS) as described previously [6, 16] with minor modifications. Briefly, 5 μl serum was incubated at 4°C overnight with 25 μl assay buffer (0.05% Triton X100 and 0.1% Tween 20 in TBS, pH 7.2) containing 5 × 106 counts/s of luciferase-tagged TSPAN7 measured in a MicroBeta LumiJET (PerkinElmer, Waltham, MA, USA). The mixture was then transferred in to a 96-well microfiltration plate (Merck Millipore, Darmstadt, Germany). Then, 15 μl Protein A-agarose (Invitrogen, Carlsbad, CA, USA) and 5 μl assay buffer containing 0.1% IgG-free BSA (Yeasen, Shanghai, China) were added to each well, and the plates were shaken (300 rev/min) at 4°C for 60 min. Plates were washed ten times using 200 μl assay buffer and Renilla luciferase substrate (Promega) was added. For potential positive serum, a competition assay was performed to confirm the binding specificity of the serum antibodies to TSPAN7. Briefly, sera were simultaneously incubated with or without luciferase-free TSPAN7.
The final results were expressed as TSPAN7A index (sample CPS – negative standard CPS) / (positive standard CPS – negative standard CPS), where CPS is counts/s. Serum that was negative for TSPAN7A was obtained from one healthy individual and serum that was positive for TSPAN7A was obtained from one individual newly diagnosed with type 1 diabetes. Based on the 99th percentile of 199 healthy participants, the cut-off value of TSPAN7A was 0.024. Intra-assay and inter-assay CV was in the range 6.3–12.4% and 14.9–35.7%, respectively.
Statistical analysis
Statistical analysis was performed with SPSS software version 19 (IBM, New York, NY, USA). The data are presented as the mean ± SD or medians (25th–75th percentile). A normality test was performed before the data analysis. For non-normal data, logarithmic transformation was conducted. ANOVA was used to compare normal variables among groups. Non-parametric test was performed by Mann–Whitney assay. A χ2 test was used to compare categorical variables. A McNemar test was used to determine whether TSPAN7A could increase the fraction of multiple-antibody-positive individuals. Binary logistic regression was performed to investigate possible risk factors for worsening islet function. A generalised linear mixed model was performed to compare the repeated measures data. There was no imputation on missing values. A p value <0.05 was considered statistically significant.
Results
Addition of testing for TSPAN7A helps to identify multiple-antibody positivity in individuals with LADA
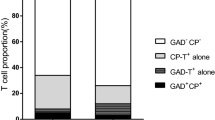
All previous studies on TSPAN7A have been conducted in white people. However, whether TSPAN7A are valid islet autoantibodies for East Asian populations remains unknown. Therefore, we first evaluated the frequency of TSPAN7A in individuals with LADA, type 1 diabetes and type 2 diabetes and in healthy control individuals. Potential positivity of sera for TSPAN7A was further confirmed in a competitive binding assay (electronic supplementary material [ESM] Fig. 1). Our results showed that the prevalence of TSPAN7A in individuals with LADA, type 1 diabetes and type 2 diabetes and healthy control individuals was 21.4% (37/173), 26% (41/158), 0.5% (1/204) and 1.2% (2/170), respectively (Table 1). The prevalence of TSPAN7A in type 1 diabetes (26%) is comparable with the data reported by Walther et al (27%; p > 0.05) using the same assay [6]. In individuals with LADA, the prevalence of GADA, IA-2A, ZnT8A and TSPAN7A was 95.4%, 17.9%, 16.8% and 21.4%, respectively (Table 1 and Fig. 1a). In individuals with type 1 diabetes, the frequency of GADA, IA-2A, ZnT8A and TSPAN7A was 69%, 43%, 31% and 26%, respectively (Table 1 and Fig. 1b). As for GADA, IA-2A and ZnT8A, positivity for TSPAN7A significantly decreased during disease progression (Fig. 1c). Comparing the prevalence of TSPAN7A in the presence or absence of other autoantibodies showed that there was a significant association of TSPAN7A with IA-2A and ZnT8A in individuals with either LADA or type 1 diabetes (all p < 0.01); there was no association between TSPAN7A and GADA (ESM Fig. 2). All the data strongly suggest that TSPAN7A are valid islet autoantibodies for East Asian populations and might be valuable in the diagnosis of autoimmune diabetes.
Association of TSPAN7A with other autoantibodies in autoimmune diabetes. (a) Association of TSPAN7A with other autoantibodies in LADA. (b) Association of TSPAN7A with other autoantibodies in type 1 diabetes. (c) Prevalence of TSPAN7A in Chinese individuals with type 1 diabetes of different disease. ***p<0.001, less vs more than 1 year duration (d) Prevalence of multiple-antibody-positive LADA and type 1 diabetes in individuals with or without TSPAN7A. ***p<0.001, with vs without TSPAN7A. Abs, autoantibodies; T1D, type 1 diabetes
We next evaluated whether TSPAN7A could improve the diagnosis of autoimmune diabetes in Chinese individuals with type 1 diabetes. Among 29 individuals with type 1 diabetes who were negative for GADA, IA2A and ZnT8A, four (13.8%) were positive for TSPAN7A. The addition of measurement of TSPAN7A slightly increased the identification of beta cell autoimmunity from 81.6% to 84.2% (p > 0.05) (Fig. 1b). Adding measurement of TSPANA7A also slightly increased the number of individuals with type 1 diabetes found to have multiple-antibody positivity, from 43.7% (69/158) to 46.2% (73/158), an increase of 5.8% (p > 0.05) (Fig. 1b,d). These results were consistent with previous reports showing that TSPAN7A provided minor utility in the diagnosis of type 1 diabetes [6]. In contrast, additional measurement of TSPAN7A significantly increased the percentage of individuals with LADA found to have multiple-antibody positivity, from 22% (38/173) to 32.4% (56/173) (an increase of 47.3%, p < 0.001) (Fig. 1a,d).
Positivity for TSPAN7A is associated with poor beta cell function
Previous studies have suggested that the presence of multiple islet autoantibodies is associated with rapid loss of beta cell function [17]. Therefore, we next investigated whether TSPAN7A were associated with low C-peptide levels in individuals with LADA in the cross-sectional study. Compared with individuals who had high C-peptide levels, those with poorer beta cell function had a lower BMI (p < 0.001), a higher GADA titre (p < 0.01) and a higher frequency of IA-2A (p < 0.05) and positivity for TSPAN7A (p < 0.01) (Table 2). For further analysis, sex, age at onset, duration, BMI, GADA titre, IA-2A, ZnT8A and TSPAN7A were evaluated in the logistic regression. Analysis of logistic regression demonstrated that duration of disease (OR 1.83, p = 0.019), GADA titre (OR 2.67, p = 0.009) and positivity for TSPAN7A (OR 2.87, p = 0.034) were risk factors for beta cell function, while BMI was a protective factor (OR 0.34, p = 0.001) (Table 3). We further compared C-peptide levels between single-antibody individuals who were positive and negative for TSPAN7A. Both FCP and PCP levels were decreased in individuals with LADA who were positive for TSPAN7A (p < 0.05; Fig. 2).
Positivity for TSPAN7A predicts progressive decline in beta cell function in individuals with LADA
To further confirm that TSPAN7A were risk factors for beta cell function, we followed up 41 TSPAN7A-negative and 12 TSPAN7A-positive individuals with LADA at baseline, 1, 2 and 3 years. The clinical characteristics, including sex, age, duration, BMI, HbA1c, GADA titre, FPG, PPG, FCP or PCP were comparable at baseline (ESM Table 1). However, after a 3-year follow-up in individuals with LADA, both FCP and PCP decreased significantly in TSPAN7A-positive, compared with TSPAN7A-negative individuals with LADA (Fig. 3). The median annual decrease in rates of FCP and PCP in individuals with LADA who were positive for TSPAN7A was much higher than in individuals who were negative for TSPAN7A (34.6% vs 7.9%, p = 0.043; 33.2% vs 11%, p = 0.041, respectively).
FCP (a) and PCP (b) in TSPAN7A-positive or -negative individuals with LADA during the 3-year follow-up. Circles, TSPAN7A-positive (n=12); triangles, TSPAN7A-negative (n=41). Missing data in LADA without TSPAN7A group: 2 in FCP and 4 in PCP at 12 months; 3 in FCP and 4 in PCP at 24 months; 1 in PCP at 36 months. Missing data in LADA with TSPAN7A group: 1 in FCP and 1 in PCP at 24 months. Data are expressed as median. *p<0.05 and **p<0.01 vs TSPAN7A-negative group
Discussion
TSPAN7A are newly discovered islet autoantibodies in autoimmune diabetes. In this study, we confirmed that TSPAN7A are valid islet autoantibodies for East Asian populations. Using cross-sectional and longitudinal cohort studies, for the first time we demonstrate that TSPAN7A can be a valuable predictor for the progressive loss of beta cell function in individuals with LADA.
TSPAN7, a four-transmembrane glycoprotein, is difficult to properly express with in vitro transcription/translation systems, making it is unsuitable for the radioligand binding assay. In contrast, the LIPS assay offers several advantages for detecting TSPAN7A. First, it is cheaper and more convenient. More importantly, because TSPAN7 is expressed by 293T cells, a mammalian cell line, it may undergo post-translational modifications, such as glycosylation [18]. Therefore, the LIPS assay was used to test TSPAN7A in our study. Correlation assays showed that TSPAN7A were positively correlated with IA-2A and ZnT8A. One possible explanation is that TSPAN7, IA-2 and ZnT8 are beta cell secretory granule membrane proteins. When beta cells are damaged, those proteins could easily be released and cause seroreaction [19,20,21].
LADA, an important type of autoimmune diabetes, is defined as a subgroup of individuals who are independent of insulin for the first 6 months after diagnosis but have detectable islet autoantibodies [22]. We found that the prevalence of TSPAN7A in individuals with LADA was 21.4% (similar to IA-2A and ZnT8A), whereas in individuals with type 2 diabetes the prevalence was 0.5%. Considering the very low frequency of TSPAN7A in individuals with type 2 diabetes, TSPAN7A do not appear to increase the diagnostic rate of LADA.
GADA are the most widely used of the islet autoantibodies for the diagnosis of LADA because positivity for GADA is not affected by the age at diagnosis and GADA are more sensitive than IA-2A or ZnT8A [22]. However, previous studies have suggested that the majority of GADA, as well as IAA, measured by the radioligand binding assay in single-antibody-positive individuals, are low-affinity [23, 24], which does not completely reflect the degree of islet autoimmunity [25]. Several laboratories have also reported that GAD autoantibody affinity is higher in multiple-antibody-positive individuals than in single-antibody-positive individuals [25, 26], suggesting that the number of islet autoantibody types present could be an indicator of islet autoimmunity. Due to the complicated procedure involved in testing antibody affinity, it is more convenient and feasible to test for more islet autoantibodies than for antibody affinity in clinical practice on a large scale. Interestingly, our study found that the combination of TSPAN7 autoantibody measurement can improve the diagnosis of multiple-antibody-positive individuals with LADA. Therefore, it is meaningful to test for TSPAN7A in individuals with LADA to improve the identification of those with multiple islet autoantibodies.
Multiple islet autoantibodies have been reported to be associated with the rapid loss of beta cell function [27]. The detection of TSPAN7A in individuals with LADA increased the number of multiple-antibody-positive individuals detected, raising the question of whether TSPAN7A are associated with the poor beta cell function in individuals with LADA. We addressed this question using cross-sectional and follow-up studies. Because individuals with LADA who had FCP <250 pmol/l are unable to achieve glycaemic control without insulin [28], we divided participants with LADA into two groups, FCP <250 pmol/l and FCP ≥250 pmol/l, in a cross-sectional study. Logistic regression analysis indicated that TSPAN7A were risk factors for lower C-peptide levels. This was confirmed by comparing FCP and PCP levels in single-antibody-positive individuals with LADA who were positive and negative for TSPAN7A. Interestingly, ZnT8A were not associated with beta cell function in another cross-sectional study [29]. The subsequent cohort study with a 3 year follow-up proved the predictive value of TSPAN7A for beta cell function. Taken together, positivity for TSPAN7A can predict a rapid loss of beta cell function in individuals with LADA.
We also explored the diagnostic value of TSPAN7A for type 1 diabetes in a Chinese population. Although TSPAN7A exist in 26% of Chinese individuals with type 1 diabetes, they largely overlap with other islet autoantibodies and only provide a minor value in type 1 diabetes diagnosis. This is consistent with data reported by Walther et al [6]. Consistent with our previous studies [30, 31], the frequencies of GADA, IA-2A and ZnT8A were much lower in Chinese individuals with type 1 diabetes when compared with white individuals with type 1 diabetes. This may be due to differences in genetic background between races. HLA plays an essential role in type 1 diabetes susceptibility and is associated with islet autoantibodies. HLA-DR3 is associated with GADA, while HLA-DR4 is strongly associated with IA-2A [32, 33]. However, the distribution of HLA-DR3 and HLA-DR4 is lower in Chinese than in white individuals with type 1 diabetes [4], which may in part explain the low frequencies of islet autoantibodies in Chinese individuals with type 1 diabetes.
In conclusion, TSPAN7A are valuable in discriminating individuals with poor beta cell function after disease progression in LADA. There are several potential limitations of our study. The number of individuals with LADA in the prospective study was relatively small and the time of follow-up was relatively short. It will be helpful to perform a longer course of study and a larger-scale randomised control trial to confirm our findings.
Abbreviations
- FCP:
-
Fasting C-peptide
- FPG:
-
Fasting plasma glucose
- GADA:
-
GAD autoantibodies
- IAA:
-
Insulin autoantibodies
- IA-2A:
-
Protein tyrosine phosphatase autoantibodies
- ICA:
-
Islet cell auto antibodies
- LADA:
-
Latent autoimmune diabetes in adults
- LIPS:
-
Luciferase immunoprecipitation system assay
- PPG:
-
2 h Postprandial plasma glucose
- PCP:
-
2 h Postprandial C-peptide
- TSPAN7:
-
Tetraspanin 7
- TSPAN7A:
-
Tetraspanin 7 autoantibodies
- ZnT8A:
-
Zinc transporter 8 autoantibodies
References
Zhou Z, Xiang Y, Ji L et al (2013) Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes 62(2):543–550. https://doi.org/10.2337/db12-0207
Mishra R, Chesi A, Cousminer DL et al (2017) Relative contribution of type 1 and type 2 diabetes loci to the genetic etiology of adult-onset, non-insulin-requiring autoimmune diabetes. BMC Med 15(1):88. https://doi.org/10.1186/s12916-017-0846-0
Zhao Y, Yang L, Xiang Y et al (2014) Dipeptidyl peptidase 4 inhibitor sitagliptin maintains β-cell function in patients with recent-onset latent autoimmune diabetes in adults: one year prospective study. J Clin Endocrinol Metab 99(5):E876–E880. https://doi.org/10.1210/jc.2013-3633
Luo S, Lin J, Xie Z et al (2016) HLA genetic discrepancy between latent autoimmune diabetes in adults and type 1 diabetes: LADA China study No. 6. J Clin Endocrinol Metab 101(4):1693–1700. https://doi.org/10.1210/jc.2015-3771
McLaughlin KA, Richardson CC, Ravishankar A et al (2016) Identification of tetraspanin-7 as a target of autoantibodies in type 1 diabetes. Diabetes 65(6):1690–1698. https://doi.org/10.2337/db15-1058
Walther D, Eugster A, Jergens S et al (2016) Tetraspanin 7 autoantibodies in type 1 diabetes. Diabetologia 59(9):1973–1976. https://doi.org/10.1007/s00125-016-3997-1
Menager MM (2017) TSPAN7, effector of actin nucleation required for dendritic cell-mediated transfer of HIV-1 to T cells. Biochem Soc Trans 45(3):703–708. https://doi.org/10.1042/BST20160439
Hald J, Galbo T, Rescan C et al (2012) Pancreatic islet and progenitor cell surface markers with cell sorting potential. Diabetologia 55(1):154–165. https://doi.org/10.1007/s00125-011-2295-1
Aanstoot HJ, Kang SM, Kim J et al (1996) Identification and characterization of glima 38, a glycosylated islet cell membrane antigen, which together with GAD65 and IA2 marks the early phases of autoimmune response in type 1 diabetes. J Clin Invest 97(12):2772–2783. https://doi.org/10.1172/JCI118732
Winnock F, Christie MR, Batstra MR et al (2001) Autoantibodies to a 38-kDa glycosylated islet cell membrane-associated antigen in (pre)type 1 diabetes: association with IA-2 and islet cell autoantibodies. Diabetes Care 24(7):1181–1186. https://doi.org/10.2337/diacare.24.7.1181
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15(7):539–553. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
Deng C, Xiang Y, Tan T et al (2016) Altered peripheral B-lymphocyte subsets in type 1 diabetes and latent autoimmune diabetes in adults. Diabetes Care 39(3):434–440. https://doi.org/10.2337/dc15-1765
Huang G, Xiang Y, Pan L, Li X, Luo S, Zhou Z (2013) Zinc transporter 8 autoantibody (ZnT8A) could help differentiate latent autoimmune diabetes in adults (LADA) from phenotypic type 2 diabetes mellitus. Diabetes Metab Res Rev 29(5):363–368. https://doi.org/10.1002/dmrr.2396
Huang G, Yin M, Xiang Y et al (2016) Persistence of glutamic acid decarboxylase antibody (GADA) is associated with clinical characteristics of latent autoimmune diabetes in adults: a prospective study with 3-year follow-up. Diabetes Metab Res Rev 32(6):615–622. https://doi.org/10.1002/dmrr.2779
Burbelo PD, Hirai H, Issa AT et al (2010) Comparison of radioimmunoprecipitation with luciferase immunoprecipitation for autoantibodies to GAD65 and IA-2β. Diabetes Care 33(4):754–756. https://doi.org/10.2337/dc09-1938
Ling Y, Jiang P, Li N, Yan Q, Wang X (2018) A luciferase immunoprecipitation assay for the detection of proinsulin/insulin autoantibodies. Clin Biochem 54:51–55. https://doi.org/10.1016/j.clinbiochem.2018.02.011
Lampasona V, Petrone A, Tiberti C et al (2010) Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: non insulin requiring autoimmune diabetes (NIRAD) 4. Diabetes Care 33(1):104–108. https://doi.org/10.2337/dc08-2305
Burbelo PD, Hirai H, Leahy H et al (2008) A new luminescence assay for autoantibodies to mammalian cell-prepared insulinoma-associated protein 2. Diabetes Care 31(9):1824–1826. https://doi.org/10.2337/dc08-0286
Lindskog C, Asplund A, Engkvist M, Uhlen M, Korsgren O, Ponten F (2010) Antibody-based proteomics for discovery and exploration of proteins expressed in pancreatic islets. Discov Med 9:565–578
Notkins AL, Zhang B, Matsumoto Y, Lan MS (1997) Comparison of IA-2 with IA-2β and with six other members of the protein tyrosine phosphatase family: recognition of antigenic determinants by IDDM sera. J Autoimmun 10(3):245–250. https://doi.org/10.1006/jaut.1997.0132
Chimienti F, Devergnas S, Favier A, Seve M (2004) Identification and cloning of a β-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53(9):2330–2337. https://doi.org/10.2337/diabetes.53.9.2330
Buzzetti R, Zampetti S, Maddaloni E (2017) Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol 13(11):674–686. https://doi.org/10.1038/nrendo.2017.99
Miao D, Guyer KM, Dong F et al (2013) GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes 62(12):4174–4178. https://doi.org/10.2337/db13-0534
Yu L, Miao D, Scrimgeour L, Johnson K, Rewers M, Eisenbarth GS (2012) Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes 61(1):179–186. https://doi.org/10.2337/db11-0670
Krause S, Landherr U, Agardh CD et al (2014) GAD autoantibody affinity in adult patients with latent autoimmune diabetes, the study participants of a GAD65 vaccination trial. Diabetes Care 37(6):1675–1680. https://doi.org/10.2337/dc13-1719
Mayr A, Schlosser M, Grober N et al (2007) GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 56(6):1527–1533. https://doi.org/10.2337/db06-1715
Juusola M, Parkkola A, Harkonen T et al (2016) Positivity for zinc transporter 8 autoantibodies at diagnosis is subsequently associated with reduced β-cell function and higher exogenous insulin requirement in children and adolescents with type 1 diabetes. Diabetes Care 39(1):118–121. https://doi.org/10.2337/dc15-1027
Jones AG, Hattersley AT (2013) The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med 30(7):803–817. https://doi.org/10.1111/dme.12159
Andersen MK, Harkonen T, Forsblom C, Groop PH, Knip M, Tuomi T (2013) Zinc transporter type 8 autoantibodies (ZnT8A): prevalence and phenotypic associations in latent autoimmune diabetes patients and patients with adult onset type 1 diabetes. Autoimmunity 46(4):251–258. https://doi.org/10.3109/08916934.2012.741155
Wang JP, Zhou ZG, Lin J et al (2007) Islet autoantibodies are associated with HLA-DQ genotypes in Han Chinese patients with type 1 diabetes and their relatives. Tissue Antigens 70(5):369–375. https://doi.org/10.1111/j.1399-0039.2007.00916.x
Yang L, Luo S, Huang G et al (2010) The diagnostic value of zinc transporter 8 autoantibody (ZnT8A) for type 1 diabetes in Chinese. Diabetes Metab Res Rev 26(7):579–584. https://doi.org/10.1002/dmrr.1128
Gorus FK, Balti EV, Messaaoui A et al (2017) Twenty-year progression rate to clinical onset according to autoantibody profile, age, and HLA-DQ genotype in a registry-based group of children and adults with a first-degree relative with type 1 diabetes. Diabetes Care 40(8):1065–1072. https://doi.org/10.2337/dc16-2228
Krischer JP, Lynch KF, Schatz DA et al (2015) The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 58(5):980–987. https://doi.org/10.1007/s00125-015-3514-y
Acknowledgements
The authors thank E. Bonifacio (DFG Research Center for Regenerative Therapies Dresden, Technische Universität Dresden, Dresden, Germany) for his support in the establishment of LIPS in our laboratory. Thanks also go to the investigators for following up the individuals.
Funding
This study was supported by the National Key R&D Program of China (2018YFC1315603, 2016YFC1305000, 2016YFC1305001), the National Science and Technology Infrastructure Program (2015BAI12B13), the National Natural Science Foundation of China (81461168031, 81500600, 81670716), the Key Project of Chinese Ministry of Education (113050A) and Xiangya Medical Big Data of Central South University.
Author information
Authors and Affiliations
Contributions
XS conducted the experiments, analysed data and wrote the manuscript. GH contributed to the experiments and study design and to the discussion and revision of the manuscript. ZL and XL analysed the data and critically edited the manuscript. CD and YW contributed to the data analysis and to drafting and revision of the manuscript. PZ and ZZ designed the study and contributed to the discussion and the critical editing of the manuscript. All authors read and approved the final manuscript. PZ and ZZ are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 138 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shi, X., Huang, G., Wang, Y. et al. Tetraspanin 7 autoantibodies predict progressive decline of beta cell function in individuals with LADA. Diabetologia 62, 399–407 (2019). https://doi.org/10.1007/s00125-018-4799-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4799-4