Abstract
Cardiovascular disease is the leading cause of death in both men and women. This is also true for patients with diabetes. In general, differences between the sexes are present in several areas, such as epidemiology, pathophysiology, diagnostics, treatment response and prognosis, as well as the way in which disease is experienced and expressed. Cardiovascular disease presents later in life in women, who are therefore more likely to suffer from comorbidities. However, this age-related difference is attenuated in women with diabetes, who suffer their first myocardial infarction at about the same age as men with diabetes. Diabetes mellitus increases the risk of cardiovascular disease by three to four times in women and two to three times in men, after adjusting for other risk factors. This paper describes the differences in cardiovascular disease in men and women and the special situation of women with type 2 diabetes when it comes to risk factors, symptoms and the setting of acute coronary syndromes. Furthermore, it highlights the importance of sex-specific analyses in clinical research to improve our knowledge of cardiovascular disease in women in general and in women with diabetes in particular. The importance of taking sex into account when treating women and men at risk of cardiovascular disease is discussed.
Similar content being viewed by others
Sex aspects in cardiovascular disease
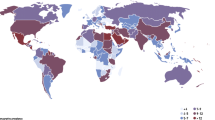
Despite a remarkable improvement in life expectancy and a decline in cardiovascular mortality during the last few decades, cardiovascular disease (CVD) is still the leading cause of death in men and women (Fig. 1) [1]. It is estimated that CVD causes 35% of all mortality in the world [1] and 48% of all deaths in Europe [2]. The major causes of CVD are ischaemic heart disease and stroke. Ageing populations and improved CVD survival will increase morbidity and step up the demands imposed on healthcare systems in the future. CVD has traditionally been perceived as a male illness but, in Europe—with the exceptions of France, the Netherlands and Spain—at least, it ends the lives of as many women as men [2], and worldwide, CVD is the most common cause of death in women in most countries except for Africa, especially above 60 years of age [3]. The ongoing global epidemics of obesity and diabetes will further accelerate this problem [4]. Women’s health has traditionally focused on matters related to sexual and reproductive health. The lack of awareness of CVD in women, among both clinicians and women themselves, is especially alarming in countries with low or intermediate incomes, where public-health policy has largely focused on infectious diseases in general and the maternal and reproductive health of women in particular.
Mortality rates for ischaemic heart disease, 2009 (or the nearest year), in men and women. Reproduced with permission from [1]. Dark blue, women; light blue, men. OECD, Organisation for Economic Co-operation and Development (countries)
Another important fact is that as many as six in every ten deaths from CVD are related to modifiable risk factors, even among women [3]. It is therefore critically important that we increase the awareness of the sex aspects of CVD among healthcare providers and the general public. There are, for example, sex differences in the impact of conventional risk factors and in the presentation of an acute coronary event. It is particularly important that the situation of women with diabetes is communicated so that events can be prevented more effectively. Even though the cardiology community has increased attention on women with CVD during the last 10 to 15 years, using campaigns such as ‘Go red for women’ and publishing sex-related guidelines [5–7], the evidence to support the treatment of heart disease in women remains incomplete [8, 9] and awareness is still not optimal. In Sweden, a survey from 2006 at the start of the Swedish ‘Go red’ campaign revealed that about 18% of the 1,000 participants recognised CVD as the main killer in women. In March 2012, the same type of survey initiated by the Swedish Heart and Lung Foundation reported that this figure had increased to 40%, although 60% did not know [10].
By systematically increasing sex-specific analyses in future cardiovascular research, our knowledge and understanding of the differences between the sexes in terms of epidemiology and treatment will increase. This article discusses the particular risk seen in women with type 2 diabetes. For information on women with type 1 diabetes, we recommend the update by Nadeau and Reusch [11].
Diabetes and CVD
People with diabetes run a high risk of CVD and mortality, and CVD is a major cause of death [4, 12]. There has been a welcome decline in CVD deaths in both men and women in Western Europe and North America in recent decades, but the same trend has not been seen in Central or Eastern Europe [2, 13, 14]. However, this decline has not been observed to the same extent in people with diabetes [15, 16], and some studies [15, 16], but not all [17], report that this is particularly the case for women with diabetes. The longer life expectancy in general might be one explanation. In Sweden, life expectancy has increased in people with diabetes [13], and a recent report from the USA showed a significant improvement in the estimated 10-year risk of CHD among adults with diabetes [18]. A large number of the global adult population suffer from glucose abnormalities, and the global trends indicate an increase in prevalence of epidemic proportions [4]. Ageing populations and lifestyle changes, with increased obesity, reduced physical activity and changes in dietary habits, are some of the explanations. This epidemic will not only have diabetes-related impacts on personal lives and healthcare costs, it will also have a major effect on the future panorama of CVD and healthcare. The global prevalence of individuals living with diabetes is expected to increase from about 8.3% in 2011 to 9.9% by 2030 [4]. Moreover, a large number of people with undiscovered diabetes or impaired glucose tolerance also run an increased risk of future CVD mortality [19, 20]. According to the most recent Diabetes Atlas [4], women account for about 50% of people living with diabetes. There is no clear evidence that the prevalence of diabetes differs between men and women, despite a reported male-to-female ratio varying widely between different countries and ethnic groups.
Impact of CVD risk factors in men and women with and without diabetes, and the sex gap
According to the INTERHEART study report from 2004, nine factors are responsible for 90% of all myocardial infarctions [21]. One of these is diabetes and the others are dyslipidaemia, hypertension, smoking, psychosocial stress, obesity—especially abdominal fat—physical inactivity, poor eating habits with too little fruit, and high alcohol intake. Diabetes increases the risk of CVD and mortality by two to four times. However, diabetes has a different impact in women and men; it increases the risk by about four times in women and about twofold in men [21, 22]. The combination of several risk factors further enhances the risk [21, 22] and such combinations are common in patients with type 2 diabetes, who often have a cluster of risk factors related to the metabolic syndrome. CVD presents about 10 years later in women than in men [23], but this age gap is almost completely attenuated when diabetes is present; it is therefore possible to conclude that women with diabetes lose their normal ‘female’ protection from CVD. One consequence is that women with diabetes suffer myocardial infarctions several years earlier than women without diabetes and thus approaching the same age of onset for myocardial infarction as men [13, 15, 22].
These findings of increased cardiovascular risk in women vs men with diabetes have been interpreted in contradicting ways by different investigators and have been extensively analysed and discussed, for example, in meta-analyses by Huxley et al, Kanaya et al and Orchard [22, 24, 25]. Some did not find an increased risk while others found an increased risk solely related to increased risk-factor burden and comorbidity in women compared with men with diabetes. Others found that increased risk remained in women with diabetes, even after adjusting for other risk factors. In the meta-analysis by Huxley et al, the increased age-adjusted relative risk of CHD was more extensively reduced in women, from 3.69 to 3.12, compared with men, in whom it fell from 2.16 to 1.99, after adjusting for multiple risk factors such as hypertension, lipid abnormalities and weight [22]. Accordingly, even after adjustment for a more severe cardiovascular risk profile, they found a somewhat higher remaining risk of CVD in women than in men with diabetes. Thus, sex itself might be a contributor, a fact that needs to be further confirmed and understood. However, the authors also discussed the possible explanation of less aggressive preventive treatment in women with diabetes. When it comes to absolute cardiovascular event rates at younger ages, the numbers are higher in men compared with women, regardless of whether or not they have diabetes [13, 26–28].
The reasons for the lower risk of CVD in women without diabetes at younger ages and the reason why this protection is attenuated when women develop diabetes are still not understood and might be a clue to understanding the differences in the age distribution of CVD between the sexes. One important factor might be sex differences in the impact of insulin resistance, which also appears to be differentially influenced by age, sex hormones and lifestyle factors in men and women [28, 29]. Another reason might be a difference in the impact of existing and clustering risk factors, including hormonal levels [23, 30]. In fact, the sex difference for age at first myocardial infarction in the general population has largely been explained by the higher levels of risk factors at younger ages in men compared with women [23], such as blood lipids, smoking and family history. Indeed, a recent paper in Diabetologia reported that the insulin-resistant state attenuated the otherwise more favourable risk profile seen in women without diabetes [31]. The role of sex hormones in this context needs to be explored more closely.
In women, risk factors considered to be related to early CVD in the absence of known diabetes are polycystic ovary syndrome, premature menopause, gestational diabetes and hypertension, as well as a history of pre-eclampsia [32, 33]. Recently, lactation has emerged as a factor related to CVD: a longer lactation period appears to have a protective effect when it comes to future CVD [34]. A sex analysis from the INTERHEART study found that several risk factors (such as hypertension, diabetes, high alcohol intake and low physical activity) were stronger in women than in men. Diabetes, current smoking, hypertension, apolipoprotein (apo)B/A levels, smoking and hypertension were more strongly associated with myocardial infarction in women below the age of 60 compared with older women [23]. Increased LDL-cholesterol levels are related to CVD in both men and women, but low HDL-cholesterol and increased triacylglycerol levels have been shown to be more strongly related to CVD in women than in men [35]. The Women’s Health Study recently reported that, in the presence of a low total-atherogenic-particle burden, as defined by an apoB100 level <0.90 g/l, few CV events occurred and there was no remaining association between CV events and HDL-cholesterol level [36]. Unfortunately, only a small percentage of the women had diabetes, but diabetes was more common among those who, despite high HDL-cholesterol levels, had a CV event. In general, women from a young age to menopause have lower LDL-cholesterol levels and higher levels of HDL-cholesterol and apoA-I than men [37]. However, on entering the menopause, women’s total and LDL-cholesterol levels increase to higher levels than in men [30, 37]. The typical lipid profile in patients with type 2 diabetes is normal or there are low levels of LDL- and HDL-cholesterol and increased triacylglycerol levels. Sex differences in lipid profiles when diabetes is present are less well explored but, regardless of this, both primary and secondary intervention trials significantly reduce CVD in men and women with diabetes and with at least one cardiovascular risk factor [38–40].
Importantly, as discussed above, when it comes to women with known diabetes, the risk of CVD is increased even after taking account of other risk factors [22]. This indicates that the diabetes itself is extremely important in women. The risk-score system from the Framingham study [41] and in Europe, the Systematic COronary Risk Evaluation (SCORE) system [42] use classic risk factors for the evaluation of CVD risk. In patients with diabetes, the UKPDS risk engine is available [43]. Both the Framingham study and the European SCORE system have limitations in women aged 65 years or older, and underestimate the true risk for elderly women. The diabetes UKPDS engine has the advantage of including important diabetes-related factors known to increase the risk of CHD, such as glucose control and duration of diabetes. However, even the UKPDS risk engine is based on a diabetes population under 65 years of age, and might therefore underestimate the true risk for elderly women. Furthermore, it is important to remember that female sex is rated as a beneficial risk factor in comparison with male sex in the UKPDS engine, and the engine therefore fails to take account of the considerably increased risk women with diabetes run in relation to women without diabetes. The recently developed Reynolds Risk Score [44] is an attempt to improve risk-score systems in women, but further validation is needed in women with diabetes.
Psychosocial stress is nowadays a well-known independent risk factor for CVD. In the INTERHEART study, 30% of CVD risk was attributed to stress. Clinical experience shows that psychosocial stress has a different impact on CVD risk in women compared with men [45, 46], examples being that family stress is a stronger risk factor for CVD for women than for men, and that broken heart syndrome (takotsubo)—the ultimate manifestation of stress, leading to a particular form of acute heart failure—almost only occurs in women. Furthermore, some studies have reported that psychosocial work stress is independently linked to a higher risk of future type 2 diabetes, with the effect being consistently higher in women than in men [47]. Also, work stress has been linked to future weight gain in obese men in contrast to a link with weight loss in lean men. A recent sex analysis from the White Hall II study showed that sex and body-weight status play a critical role in determining the direction of the association between psychosocial stress and future type 2 diabetes, and that work stress is associated with a lower risk of type 2 diabetes among non-obese men, but with a higher risk of diabetes among obese women [48].
Glucose as a risk factor for CVD
Blood glucose level is currently regarded as a continuous risk factor for CVD; not only people with overt diabetes run an increased risk of future CVD, but also people with slightly elevated fasting or postprandial glucose levels [19, 20]. It appears that post-load glucose levels are even more important than fasting glucose levels in terms of future cardiovascular mortality, at least in men [20]. One major drawback from a sex perspective is that several of the older observational trials involving glucose levels and future CVD in groups primarily included men: the Helsinki Policemen’s Study; the first Whitehall Study; the Paris Prospective Study; and the Honolulu Heart Programme. As a result, the related meta-analyses include only a small number of women [19]. From the Framingham offspring data, it was recently reported that slightly elevated fasting glucose levels (impaired fasting glucose) were shown to be an even stronger predictor of CHD in women than in men and were almost as strongly predictive as diabetes. The question of whether lower cut-off points for fasting should be used in women in order to diagnose diabetes or impaired fasting glucose was consequently discussed [49]. It is necessary to analyse whether different cut-off points for men and women could also be extended to other risk factors, such as BMI and hypertension, as is already the case when it comes to HDL-cholesterol and triacylglycerol levels. In addition, the above discussion of stronger risk factors in younger women further underlines the importance of this question.
One major problem is that impaired glucose tolerance and type 2 diabetes often remain silent and are first discovered at the time of a cardiovascular event. In fact, when an OGTT is performed in non-diabetic patients with a myocardial infarction, as many as two-thirds are found to have impaired glucose tolerance or diabetes [50–52]. A sex analysis in the Euro Heart Survey on diabetes and the heart revealed a higher prevalence of glucose abnormalities in women than in men and only 19% of the women and 27% of the men with coronary artery disease had normal glucose regulation when tested with an OGTT [53]. These previously undiscovered glucose disturbances are also adversely related to outcome of myocardial infarction [54, 55]. In the recent report on HbA1c levels in the INTERHEART study [56], self-reported diabetes did indeed underestimate the true prevalence of dysglycaemia. Furthermore, after adjusting for the nine known risk factors applied in the study (including diabetes and sex), HbA1c remained a progressive independent predictor of myocardial infarction in patients with and without diabetes and was related to increased risk in both men and women (OR for men 1.38 [95% CI 1.32, 1.43] and for women 1.45 [1.36, 1.56]). Diabetes was an even stronger risk factor in women, especially those under the age of 60 [23].
To the best of our knowledge, the question of whether improved glucose levels and the use of different glucose-lowering substances have a different impact on CVD in women and men has not been extensively analysed, but performing sex-specific analyses of this kind could be of interest in the glucose intervention trials that are currently in progress. In the original paper from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, in which 38% of participants were women, there were no differences in the effect of intense glucose control on mortality or the primary endpoint between men and women (p = 0.92 and p = 0.74, respectively) [57]. Neither was there in the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) trial, in which 42% were women, any heterogeneity between men and women (p > 0.10 for heterogeneity) [58]. In the post-myocardial infarction trial Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 2, wider sex-specific analyses were performed; these failed to reveal any differences in the effect of the randomised treatment, but did identify different risk-factor profiles in women and men and, in general, a heavier risk-factor burden in women. The presence of diabetes complications was, in particular, a negative predictor for CVD after myocardial infarction in women [59].
Sex-specific conditions in women
Several women-specific conditions can complicate and influence the risk of future CVD, such as polycystic ovary syndrome, premature menopause, gestational diabetes and history of pre-eclampsia. In pregnant women with diabetes, type 1 or type 2, the risk of accelerating diabetes complications is another obvious risk, in addition to the risk of fetal malformation. Planned pregnancy with optimised glucose control is important for both the child and the mother. However, the risk also extends to women with elevated glucose levels during pregnancy, as recently shown in the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study [60]. Up to 18% of pregnant women without known diabetes could have gestational diabetes if tested with an OGTT. New proposed guidelines from the International Association of Diabetes and Pregnancy Study Groups (IADPSG; www.iadpsg.org) on diagnostic criteria and screening for gestational diabetes have been published recently and aim to predict an adverse pregnancy outcome for the child and the mother [61, 62]. For the mother, it is not only the risk of future diabetes that is present but also an increased risk of CVD. A recent report from Canada indicates that women with gestational diabetes run a particularly high risk of future CVD and a substantial part of this risk is attributed to the subsequent development of type 2 diabetes, highlighting the importance of diabetes prevention in this group [63]. Consequently, the prevention of weight gain and abdominal obesity after pregnancy appears to be very important as, particularly in younger women, these risk factors are related to future CVD.
In women with diabetes or polycystic ovary syndrome, there is a hormonal imbalance in androgen and oestrogen levels, and sex hormones appear to influence glucose control in women with diabetes [64]. Promising experimental research results, including the positive effects of oestrogen on endothelial function [65] and the obvious clinical relief of typical menopausal symptoms, were the basis of previously extensive use of sex-hormone-replacement therapy in postmenopausal women. Randomised intervention trials with hormone replacement, such as the Heart and Estrogen/Progestin Replacement Study (HERS) and the Women’s Health Initiative (WHI) trial, did not, however, show any beneficial effects on CVD, but instead indicated a slightly increased risk of thrombo-embolic events and breast cancer [66]. The question of whether treatment with hormone-replacement therapy was started too late after menopause has since been discussed. No specific diabetes data have been presented in the WHI studies. In overall terms, the use of postmenopausal hormone therapy has decreased markedly [67].
Acute coronary syndromes in women and diabetes
There has been a substantial improvement in outcome after myocardial infarction in patients with and without diabetes during the last few years. The results from the Swedish register of coronary care (RIKS-HIA, SWEDEHEART), which includes almost all patients admitted to coronary care units in Sweden, showed an absolute reduction in the 1-year mortality rate after myocardial infarction of 10% from 1995 to 2002 in patients with diabetes (from 29.7% to 19.7% compared with 16.6% to 12.1% in those without diabetes in 1995–2002) [68] and further improvements are seen every year. Enhanced coronary care, the improved use of evidence-based treatment, such as lipid-lowering treatment and early coronary revascularisation, as well as improved risk-factor management before myocardial infarction are some explanations. However, diabetes is still an independent predictor of adverse outcome and the mortality gap after a coronary event between patients with and without diabetes increases with follow-up time, even after modern treatment techniques, such as percutaneous coronary intervention with a stent [69].
Back in 1988, data from the Framingham cohort revealed that women with diabetes had a poorer outcome after myocardial infarction than men [70]. Older myocardial infarction trials recruited, due to inclusion and exclusion criteria, limited numbers of patients with diabetes and even smaller numbers of women with diabetes, and this has hampered knowledge of the best treatment strategies for this group. In sex-specific analyses (Fig. 2) from our group reveal that at age under 65 years, women with diabetes had the poorest outcome after a coronary event, even worse than men with diabetes [71], a finding that was not apparent before the sex-stratified analyses. The increased risk was explained by an elevated burden of risk factors, including hypertension, heart failure and smoking, that was already present at admission and was not related to sex itself or to any major differences in evidence-based treatment. Despite this, treatment with ACE inhibitors was shown to be less common in women with diabetes after adjusting for other risk factors. Further evaluations are needed to explain why these younger women with diabetes more commonly had an excess of risk factors at admission compared with men with diabetes and women without diabetes. The higher risk-factor burden at admission may be the natural result of sex-related vulnerability to risk factors in younger women, and women with diabetes and glucose disturbances appear to have more adverse metabolic disturbances than men [22, 23, 52, 72–74]. Whether earlier and more aggressive risk-factor intervention in women with diabetes can prevent CVD complications needs further evaluation. One possible explanation for the higher prevalence of heart failure could be a sex difference in previous undiagnosed heart failure or previous silent myocardial ischaemia. It is claimed that women, and especially women with diabetes, suffer more frequently from heart failure with preserved ejection fraction (‘diastolic dysfunction’), a condition known to be widely undiagnosed in the general population [75]. Furthermore, symptoms of myocardial ischaemia might more frequently be atypical in patients with diabetes as a result of autonomic dysfunction and a lower perception or lack of pain, resulting in only tiredness and breathlessness [76]. For this reason, there is a risk of undiagnosed previous silent myocardial infarctions and coronary ischaemia in patients with diabetes. One result might be fewer referrals for investigation and less aggressive treatment, with fewer revascularisations.
Analyses of mortality after myocardial infarction in patients with diabetes in Sweden (RIKS-HIA, SWEDEHEART) in 1995–2002, by sex. Adapted from [71]. n = 70,882; 31% women; 21% with diabetes mellitus. a Age ≤64 years, RR for women with diabetes 1.35 (95% CI 1.18, 1.54) and women without diabetes 0.96 (95% CI 0.86, 1.07); (b) age 65–74 years, RR for women with diabetes 0.99 (95% CI 0.91, 1.08) and women without diabetes 0.88 (95% CI 0.83, 0.94); and (c) age ≥75 years, RR for women with diabetes 0.96 (95% CI 0.89, 1.04) and women without diabetes 0.87 (95% CI 0.83, 0.91). Red broken line, non-diabetic women; red solid line, women with diabetes; blue broken line, non-diabetic men; and blue solid line, men with diabetes
In general, there appears to be a sex difference in the presentation of symptoms and the way women and men express their symptoms. Men more frequently describe chest pain, left arm pain or diaphoresis, while women more commonly describe nausea, tiredness and jaw pain, although the opposite may occur. The difference in the occurrence of chest pain has been related to women’s older age and the presence of diabetes [77]. Consequently, it could be assumed that women with diabetes more frequently experience atypical or silent myocardial infarction. One other important difference between women with and without diabetes and acute coronary syndrome is the extent of atheromatosis and stenosis of the coronary arteries. Women with diabetes more frequently have significant stenosis in all three coronary arteries, to the same extent as men with diabetes, while women without diabetes more frequently have non-obstructive coronary artery disease [78].
Medical treatment and early revascularisation after an acute coronary event have consistently shown at least the same impact in patients with diabetes as in those without diabetes [68, 79]. Without further knowledge and until this subject is better explored, it can be assumed that this finding could be extended to women with diabetes, and approaches to primary and secondary prevention should be based on updated guidelines [6, 79].
Summary and future directions
Diabetes is a strong risk factor for future cardiovascular complications and in women diabetes attenuates the usual female advantage. Unfortunately, there appears to be a heavy risk-factor burden in women with diabetes, and younger women appear especially sensitive to CVD risk factors. An increase in the use of primary and secondary preventive measures to reduce the risk-factor burden and prevent or postpone coronary events is important. Aggressive multifactorial risk management, including lifestyle changes, has been shown to be important in reducing cardiovascular morbidity and mortality in patients with diabetes [80], and earlier diagnosis of coronary artery disease, before the onset of myocardial complications, is recommended. During pregnancy, gestational diabetes could easily be discovered and, if identified, the woman should be followed more intensively for the rest of her lifetime because of the risk of future CVD and diabetes. Cardiologists should be encouraged to include women’s health issues, such as pregnancy complications and use of birth control, when taking medical histories and assessing cardiovascular risk. As trials in patients with myocardial infarction have recruited a limited number of patients with diabetes and an even smaller number of women with diabetes, our knowledge of the most effective treatment and preventive strategies is limited. More research on the impact of sex in diabetes and CVD is needed, together with an awareness of the need to include sufficient numbers of women, as advocated by international guidelines [5–7]. For both men and women, it is important to have more sex-based knowledge of diabetes and CVD.
Abbreviations
- Apo:
-
Apolipoprotein
- CVD:
-
Cardiovascular disease
- SCORE:
-
Systematic COronary Risk Evaluation
- WHI:
-
Women’s Health Initiative
References
Organisation for Economic Co-operation and Development (2011) Health at a glance 2011: OECD indicators. Available from doi:10.1787/health_glance-2011-en
Scarborough P, Wickramasinghe K, Bhatnagar P, Rayner M (2011) Trends in coronary heart disease 1961–2011. British Heart Foundation, London
World Health Organization (2009) Women and health: today’s evidence tomorrow’s agenda. WHO, Geneva
International Diabetes Federation (2011). IDF Diabetes Atlas, 5th edn. IDF, Brussels. Available from www.idf.org/diabetesatlas
Mosca L, Grundy SM, Judelson D et al (1999) Guide to preventive cardiology for women: AHA/ACC Scientific Statement Consensus panel statement. Circulation 99:2480–2484
Mosca L, Benjamin EJ, Berra K et al (2011) Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation 123:1243–1262
Stramba-Badiale M, Fox KM, Priori SG et al (2006) European gender guidelines cardiovascular diseases in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J 27:994–1005
Stramba-Badiale M (2010) Women and research on cardiovascular diseases in Europe: a report from the European Heart Health Strategy (EuroHeart) project. Eur Heart J 31:1677–1681
Melloni C, Berger JS, Wang TY et al (2010) Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 3:135–142
Swedish Heart and Lung Foundation (2012) Press Release, March 8, 2012. Press contact Anna Sjödin (anna.sjodin@hjart-lungfonden.se) [press release in Swedish]
Nadeau KJ, Reusch JEB (2011) Cardiovascular function/dysfunction in adolescents with type 1 diabetes. Curr Diab Rep 11:185–192
Seshasai SR, Kaptoge S, Emerging Risk Factors Collaboration et al (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364:829–841
Swedish National Board of Health and Welfare (2009) Folkhälsorapport 2009. Swedish National Board of Health and Welfare, Stockholm [in Swedish]
Cooper R, Cutler J, Desvigne-Nickens P et al (2000) Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation 102:3137–3147
Gu K, Cowie CC, Harris MI (1998) Diabetes and decline in heart disease mortality in US adults. JAMA 281:1291–1297
Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC (2007) Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 147:149–155
Preis SR, Hwang SJ, Coady S et al (2009) Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 119:1728–1735
Ford ES (2011) Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the US. Findings from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes Care 34:1337–1343
Coutinho M, Gerstein HC, Wang Y, Yusuf S (1999) The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22:233–240
DECODE Study Group (2001) Glucose tolerance and cardiovascular mortality. Comparison of fasting and 2-hours diagnostic criteria. Arch Intern Med 161:397–405
Yusuf S, Hawken S, Ounpuu S, on behalf of the INTERHEART Study investigators et al (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet 364:937–952
Huxley R, Barzi F, Woodward M (2006) Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 332:73–78
Anand SS, Islam S, Rosengren A, on behalf of the INTERHEART Investigators et al (2008) Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 29:932–940
Kanaya AM, Grady D, Elizabeth Barrett-Connor E (2002) Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus. A meta-analysis. Arch Intern Med 162:1737–1745
Orchard TJ (1996) The impact of gender and general risk factors on the occurrence of atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Ann Med 28:323–333
Rautio A, Lundberg V, Messner T, Nasic S, Stegmayr B, Eliasson M (2005) Favourable trends in the incidence and outcome of myocardial infarction in non diabetic, but not in diabetic subjects: finding from the MONICA myocardial infarction registry in northern Sweden in 1989–2000. J Intern Med 258:369–377
Lloyd-Jones D, Adams R, Carnethon M et al (2009) American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119:e21–e181
Orchard TJ, Becker DJ, Kuller LH, Wagener DK, LaPorte RE (1982) Age and sex variations in glucose tolerance and insulin response: parallels with cardiovascular risk. J Chronic Dis 35:123–132
Mittendorfer B (2005) Insulin resistance: sex matters. Curr Opin Clin Nutr Metab Care 8:367–372
Berg G, Mesch V, Boero L et al (2004) Lipid and lipoprotein profile in menopausal transition. Effects of hormones, age and fat distribution. Horm Metab Res 36:215–220
Wannamethee PO, Lawlor DA et al (2012) Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 55:80–87
Carpenter MW (2007) Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care 30(Suppl 2):S246–S250
Hannaford P, Ferry S, Hirsch S (1997) Cardiovascular sequelae of toxaemia of pregnancy. Heart 77:154–158
Stuebe AM, Michels KB, Willett WC, Manson JE, Rexrode K, Rich-Edwards JW (2009) Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Gynecol 200:138.e1–138.e8
Hokansson JE, Austin MA (1996) Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population based prospective studies. J Cardiovasc Risk 3:213–219
Mora S, Buring JE, Ridker PM, Cui Y (2011) Association of high-density lipoprotein cholesterol with incident cardiovascular events in women, by low-density lipoprotein cholesterol and apolipoprotein b100 levels: a cohort study. Ann Intern Med 155:742–750
Bittner V (2005) Perspectives on dyslipidemia and coronary heart disease in women. J Am Coll Cardiol 46:1628–1635
Colhoun HM, Betteridge DJ, Durrington PN, on behalf of the CARDS investigators et al (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364:685–696
Heart Protection Study Collaborative Group (2004) Effects of cholesterol lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high-risk conditions. Lancet 363:757–767
ESC/EAS Guidelines for the management of dyslipidaemias. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Catapano AL, Reiner Z, DeBacker G et al. (2011) Atherosclerosis 217:3–46
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847
de Backer G, Ambrosioni E, Borch-Johnsen K et al (2003) European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 24:1601–1610
Stevens JR, Kothari V, Adler AI, Stratton IM, Holman R, on behalf of the United Kingdom Prospective Diabetes Study (UKPDS) Group (2001) The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clinical Science 101:671–679
Ridker PM, Buring JE, Rifai N, Cock NR (2007) Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297:611–619
Orth-Gomér K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA (2000) Marital stress worsens prognosis in women with coronary heart disease: The Stockholm Female Coronary Risk Study. JAMA 284:3008–3014
Wang HX, Leineweber C, Kirkeeide R et al (2007) Psychosocial stress and atherosclerosis: family and work stress accelerate progression of coronary disease in women. The Stockholm Female Coronary Angiography Study. J Intern Med 261:245–254
Heraclides A, Chandola T, Witte DR, Brunner EJ (2009) Psychosocial stress at work doubles the risk of type 2 diabetes in middle-aged women: evidence from the Whitehall II study. Diabetes Care 32:2230–2235
Heraclides AM, Chandola T, Witte DR, Brunner EJ (2012) Work stress, obesity and the risk of type 2 diabetes: gender-specific bidirectional effect in the Whitehall II study. Obesity 20:428–433
Levitzky YS, Pencina MJ, D’Agostino RB et al (2008) Impact of impaired fasting glucose on cardiovascular disease. The Framingham Heart Study. JACC 51:264–270
Norhammar A, Tenerz A, Nilsson G et al (2002) Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 359:2140–2144
Bartnik M, Rydén L, Fererari R et al (2004) Euro Heart Survey Investigators. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J 25:1880–1890
Hu DY, Pan CY, Yu JM (2006) The relationship between coronary artery disease and abnormal glucose regulation in China: the China Heart Survey. Eur Heart J 27:2573–2579
Dotevall A, Rosengren A, Bartnik M et al (2007) European Heart Survey Investigators. Sex-related aspects on abnormal glucose regulation in patients with coronary artery disease. Eur Heart J 28:310–315
Bartnik M, Malmberg K, Norhammar A, Tenerz A, Ohrvik J, Rydén L (2004) Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J 25:1990–1997
Lenzen M, Rydén L, Ohrvik J et al (2006) Euro Heart Survey Investigators. Diabetes known or newly detected, but no impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur Heart J 27:2969–2974
Gerstein HC, Islam S, Anand S et al (2010) Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: an analysis of 15,780 patients from the INTERHEART study. Diabetologia 53:2509–2517
The Action to Control Cardiovascular Risk in Diabetes Study Group, ACCORD (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
The ADVANCE Collaborative Group (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
Venskutonyte L, Malmberg K, Norhammar A, Wedel H, Rydén L (2010) Effect of gender on prognosis in patients with myocardial infarction and type 2 diabetes. J Intern Med 268:75–82
Metzger BE, Lowe LP, Dyer AR et al (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358:1991–2002
Coustan DR, Lowe LP, Metzger BE, Dyer AR; International Association of Diabetes and Pregnancy Study Groups (2010) The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol 202:654: 1–6
International Association of Diabetes and Pregnancy Study Groups (2010) International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33:676–682
Shah BR, Retnakaran R, Booth GL (2008) Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 31:1668–1669
Kim NN (2009) Sex steroid hormones in diabetes-induced sexual dysfunction: focus on the female gender. J Sex Med 6(Suppl 3):239–246
Ross RL, Serock MR, Khalili RA (2008) Experimental benefits of sex hormones on vascular function and the outcome of hormone therapy in cardiovascular disease. Curr Cardiol Rev 4:309–322
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Schenck-Gustafsson K, Brincat M, Erel CT et al (2011) Managing the menopause in the context of coronary heart disease. Maturitas 68:94–97
Norhammar A, Lindbäck J, Rydén L, Wallentin L, Stenestrand U, Register of Information and Knowledge about Swedish Heart Intensive Care Admission (RIKS-HIA) (2007) Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission. Heart 93:1577–1583
Norhammar A, Lagerqvist B, Saleh N (2010) Long-term mortality after PCI in patients with diabetes mellitus: results from the Swedish Coronary Angiography and Angioplasty Registry. EuroIntervention 5:891–897
Abbott RD, Donahue RP, Kannel WB (1988) The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA 260:3456–3460
Norhammar A, Stenestrand U, Lindbäck J, Wallentin L, Register of Information and Knowledge about Swedish Heart Intensive Care Admission (RIKS-HIA) (2008) Women younger than 65 years with diabetes mellitus are a high-risk group after myocardial infarction: a report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission (RIKS-HIA). Heart 94:1565–1570
Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M (2007) Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women. The Western New York Study. Diabetes Care 30:354–359
Dotevall A, Wilhemlsen L, Lappas G, Roensgren A (2005) Considerable disturbances of cardiovascular risk factors in women with diabetes and myocardial infarction. J Diabetes Complications 19:26–34
Haffner SM, Miettinen H, Stern MP (1997) Relatively more atherogenic coronary heart disease risk factors in prediabetic women than prediabetic men. Diabetologia 40:711–717
Remes J, Miettinen H, Reunanen A, Pyorala K (1991) Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J 12:315–321
Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V (1999) Silent myocardial ischemia in patients with diabetes. Who to screen. Diabetes Care 22:1396–1400
Arslanian-Engoren C, Patel A, Fang J et al (2006) Symptoms of men and women presenting with acute coronary syndromes. Am J Cardiol 98:1177–1181
Dotevall A, Hasdai D, Wallentin L, Battler A, Rosengren A (2005) Diabetes mellitus: clinical presentation and outcome in men and women with acute coronary syndromes. Data from the Euro Heart Survey ACS. Diabet Med 22:1542–1550
Rydén L, Standl E, Bartnik M et al (2007) Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 28:88–136
Gaede P, Lund-Andersen H, Parving HH, Pedersen O (2008) Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358:580–591
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript. Both the authors have received speaking honoraria from pharmaceutical companies.
Contribution statement
Both authors were responsible for the conception, design and drafting of the manuscript, and approved the final version for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Norhammar, A., Schenck-Gustafsson, K. Type 2 diabetes and cardiovascular disease in women. Diabetologia 56, 1–9 (2013). https://doi.org/10.1007/s00125-012-2694-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2694-y