Abstract
Key message
Seven sharp eyespot resistance QTL were detected consistently across five environments and delimited to seven DNA marker intervals, respectively, six of which were independent of plant height and heading time.
Abstract
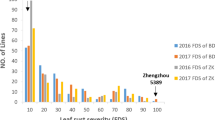
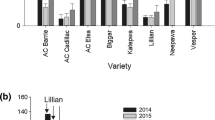
Sharp eyespot, caused mainly by the soil-borne fungus Rhizoctonia cerealis, is one of the important diseases of bread wheat (Triticum aestivum L.). This disease has escalated into a major threat to wheat production in some regions of the world. Wheat resistance to sharp eyespot can be a potential means to reduce the needs for application of fungicides and agricultural inputs. In the present study, the winter wheat lines, Luke and AQ24788-83, both of which possess quantitative resistance to sharp eyespot, were crossed and a population consisting 241 recombinant-inbred lines (RILs) was constructed. These RILs were assessed for sharp eyespot resistance by conducting five field and greenhouse trials during the period from 2008 to 2012, and they were genotyped with 549 simple-sequence repeat DNA markers. Seven quantitative trait loci (QTL) were detected consistently across the five trial environments to be associated with the sharp eyespot resistance. They were mapped on chromosomes 1A, 2B, 3B, 4A, 5D, 6B, and 7B. Four of these QTL are unequivocally novel, while it is possible that the other three might also be novel. Plant height and heading date of the 241 RILs were recorded in the four field trials. All of the seven disease resistance QTL were independent of plant height and heading time except one that was significantly associated with plant heading time. This association might be attributed genetically to a single QTL, or to different but closely linked QTL. In the case of single QTL, pleiotropism might be involved or the sharp eyespot resistance might be conferred in a physical instead of physiological nature.




Similar content being viewed by others
References
Bassam BJ, Caetano-Anolles G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Boerema GH, Verhoeven AA (1977) Check-list for scientific names of common parasitic fungi. Series 26: fungi on field crops: Cereal and grasses. Neth J Plant Pathol 83:165–204
Burpee LL, Sanders PL, Cole H Jr, Sherwood RT (1980) Aastomosis groups among isolates of Ceratobasidium cornigerum and related fungi. Mycologia 72:689–701
Cai SB, Ren LJ, Yan W, Wu JZ, Chen HG, Wu XY, Zhang XY (2006) Germplasm development and mapping of resistance to sharp eyespot (Rhizoctonia cerealis) in wheat. (In Chinese with English abstract). Sci Agric Sin 39:928–934
Chen Y, Li W, Zhang XX, Zhang BQ, Yu HS, Chen HG (2009) Composition and virulence of pathogen of wheat sharp eyespot in north latitude 33° of China. (In Chinese with English abstract). J Triticeae Crops 29:1110–1114
Clarkson JDS, Cook RJ (1983) Effect of sharp eyespot (Rhizoctonia cereatis) on yield loss in winter wheat. Plant Pathol 32:421–428
Cromey MG, Parkes RA, Fraser PM (2006) Factors associated with stem base and root diseases of New Zealand wheat and barley crops. Australas Plant Pathol 35:391–400
Guo YP, Li W, Sun HY, Wang N, Yu SH, Chen HG (2012) Detection and quantification of Rhizoctonia cerealis in soil using real-time PCR. J Gen Plant Pathol 78:247–254
Guyomarc’h H, Sourdille P, Charmet G, Edwards KJ, Bernard M (2002) Characterization of polymorphic microsatellite markers from Aegilops tauschii and transferability to the d-genome of bread wheat. Theor Appl Genet 104:1164–1172
Hamada MS, Yin YN, Ma ZH (2011) Sensitivity to iprodione, difenoconazole and fludioxonil of Rhizoctonia cerealis isolates collected from wheat in China. Crop Prot 30:1028–1033
Hammouda AM (2003) First report of sharp eyespot of wheat in Egypt. Plant Dis 87:598
Huo NX (2002) QTL analysis of resistance to diseases caused by Rhizoctonia cerealis and Blumeris graminis. (In Chinese with English abstract) Ph. D. Dissertation, Graduate School of Chinese Academy of Agricultural Sciences
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Lemańczyk G (2010) Occurrence of sharp eyespot in spring cereals grown in some regions of Poland. J Plant Prot Res 50:505–512
Lemańczyk G, Kwaśna H (2013) Effects of sharp eyespot (Rhizoctonia cerealis) on yield and grain quality of winter wheat. Eur J Plant Pathol 135:187–200
Li ZK, Pinson MSR, Marchetti MA, Stansel JW, Park WD (1995) Characterization of quantitative trait loci (QTLs) in cultivated rice contributing to field resistance to sheath blight (Rhizoctonia solam). Theor Appl Genet 91:382–388
Lipps PE, Herr LJ (1982) Etiology of Rhizoctonia cerealis in sharp eyespot of wheat. Phytopathology 72:1574–1577
Mallard S, Gaudet D, Aldeia A, Abelard C, Besnard AL, Sourdille P, Dedryver F (2005) Genetic analysis of durable resistance to yellow rust in bread wheat. Theor Appl Genet 110:401–1409
Mazzola M, Smiley RW, Rovira AD, Cook RJ (1996) Characterization of Rhizoctonia isolates, disease occurrence and management in cereals. In: Sneh B, Jabaji-Hare S, Neate S, Dijst G (eds) Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. Kluwer Academic Publishers, Dordrecht, pp 259–267
McBeath JH, McBeath J (2010) Plant diseases, pests and food security. In: Martin B (ed) Environmental change and food security in china. Springer Technology and Engineering. Springer, Dordrecht, p 136
Paillard S, Trotoux-Verplancke G, Perretant MR, Mohamadi F, Leconte M, Coëdel S, de Vallavieille-Pope C, Dedryver F (2012) Durable resistance to stripe rust is due to three specific resistance genes in French bread wheat cultivar Apache. Theor Appl Genet 125:955–965
Paterson AH, Damon S, Hewitt JD, Zamir D, Rabinowitch HD, Lincoln SE, Lander EC, Tanksley SD (1991) Resolution of Mendelian factors underlying quantitative traits in tomato: comparison across species, generations and environments. Genetics 127:181–197
Pestsova E, Ganal MW, Röder MS (2000) Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome 43:689–697
Polley RW, Thomas MR (1991) Surveys of diseases of winter wheat in England and Wales, 1976–1988. Ann Appl Biol 119:1–20
Ren LJ, Cai SB, Tang T, Wu JZ, Zhou MP, Yan W, Ma HX, Lu WZ (2004) SSR markers linked resistance QTLs to sharp eyespot (Rhizoctonia cerealis) in wheat. (In Chinese with English abstract). J Yangzhou Univ 25:16–19
Ren LJ, Zhang X, Zhou MP, Lu WZ, Ma HX (2007) QTL analysis of sharp eyespot (Rhizoctonia cerealis) and Fusarium head blight in wheat. (In Chinese with English abstract). J Triticeae Crops 27:416–420
Ren LJ, Chen PD, Chen HG, Ma HX (2010) Screening of resistance to sharp eyespot in wheat. (In Chinese with English abstract). J Plant Genet Resour 11:108–111
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal M (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Sharma A, McClung AM, Pinson SRM, Kepiro JL, Shank AR, Tabien RE, Fjellstrom R (2009) Genetic mapping of sheath blight resistance QTLs within tropical Japonica rice cultivars. Crop Sci 49:256–264
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Song QJ, Fickus EW, Cregan PB (2002) Characterization of trinucleotide SSR motifs in wheat. Theor Appl Genet 104:286–293
Sourdille P, Tavaud M, Charmet G, Bernard M (2001) Transferability of wheat microsatellites to diploid Triticeae species carrying the A, B and D genomes. Theor Appl Genet 103:346–352
Sourdille P, Cadalen T, Guyomarc’h H, Snape JW, Perretant MR, Charmet G, Boeuf C, Bernard S, Bernard M (2003) An update of the Courtot × Chinese Spring intervarietal molecular marker linkage map for the QTL detection of agronomic traits in wheat. Theor Appl Genet 106:530–538
Srinivasachary, Willocquet L, Savary S (2011) Resistance to rice sheath blight (Rhizoctonia solani Kühn) [teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk.] disease: current status and perspectives. Euphytica 178:1–22
Tang T, Ren LJ, Cai SB, Wu JZ, Lu WZ, Chen JM, Ma HX (2004) Study on QTL mapping of sharp eyespot resistance in wheat. (In Chinese with English abstract). J Triticeae Crops 24:11–16
Wang YZ, Wu ZF, Shi JR, Chen HG (1994) Study on occurrence of wheat sharp eyespot in Jiangsu province and the factors influencing its development in fields. (In Chinese with English abstract). Acta Phytophy Sin 21:109–114
Wang S, Basten JC, Zeng ZB (2010) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm. Accessed 10 Mar 2013
Wang M, Lu BL, Xing XP, Li HL (2011) Composition and virulence variation of the pathogen of wheat sharp eyespot from Henan Province. (In Chinese with English abstract). Acta Phytopathol Sin 41:556–560
Zhang XC, Li SS, Zhao XH, Fan YD, Li RJ (2005) QTL and molecular markers for resistance of wheat to sharp eyespot (In Chinese with English abstract). J Plant Genet Resour 6:276–279
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30871612), the Commonweal Specialized Research Fund of China Agriculture (200903035, nyhyzx3-16), the National Basic Research Program of China (2013CB127700), and the Program for Changjiang Scholars and Innovative Research Team (IRT1042).
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
The experiments comply with the current laws of P. R. China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Keller.
G. Li and Z. Du contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Chen, J., Li, G.H., Du, Z.Y. et al. Mapping of QTL conferring resistance to sharp eyespot (Rhizoctonia cerealis) in bread wheat at the adult plant growth stage. Theor Appl Genet 126, 2865–2878 (2013). https://doi.org/10.1007/s00122-013-2178-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-013-2178-6