Abstract
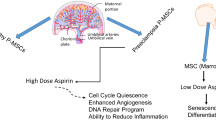
Preeclampsia (PE) is a hypertensive disorder of human pregnancy. Low-dose aspirin (acetylsalicylic acid) (60–150 mg/day) is used to prevent PE when taken early in pregnancy. The effect of aspirin on term PE remains uncertain. Abnormal placentation is a hallmark of PE and leads to increased placental oxidative stress, which triggers the release of anti-angiogenic factors that cause local damage to the decidual vasculature. The damage subsequently spreads systemically and culminates in maternal clinical symptoms. Decidua basalis mesenchymal stem/stromal cells (DMSCs) reside in a vascular microenvironment. In PE, DMSCs are exposed to abnormally high levels of oxidative stress and circulating inflammatory factors from the maternal blood. We previously showed that colony-forming unit ability and resistance to oxidative stress in DMSCs are reduced in MSCs derived from term PE pregnancies (PE-DMSCs). The action, if any, of aspirin on term PE-DMSCs has not been reported. In this study, aspirin (5 μg/mL) was found to significantly increase PE-DMSC adhesion compared to untreated PE-DMSCs and gestation-matched control DMSCs (p value < 0.001) but had no effect on PE-DMSC proliferation. ELISA analysis showed that aspirin significantly decreased the production of inflammatory cytokines IFN-γ (p value < 0.05) and IL-8 (p value < 0.001) in PE-DMSCs. In addition, aspirin treatment increased the antioxidant capacity of PE-DMSCs compared with the untreated group (p value < 0.05). This study is the first to reveal a novel, beneficial action of aspirin on PE-DMSCs from term PE pregnancies by improving their adhesion, suppressing their production of pro-inflammatory cytokines production, and increasing their antioxidant capacity.
Key messages
-
Preeclampsia (PE) is a serious hypertensive disorder of pregnancy.
-
The risk of PE is reduced by aspirin but the mechanism is poorly understood.
-
Decidua basalis mesenchymal stem/stromal cells (DMSCs) are abnormal in PE.
-
Aspirin treatment improves multiple functions of PE-DMSCs.
-
Improved DMSC function may contribute to the beneficial effect of aspirin.








Similar content being viewed by others
References
Ghulmiyyah L, Sibai B (2012) Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 36(1):56–59
Moodley J (2004) Maternal deaths associated with hypertensive disorders of pregnancy: population-based study. Hypertension Pregnancy 23(3):247–256
Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Giguère Y (2010) Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 116(2, Part 1):402–414
Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E (2013) Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstetrics Gynecology 41(5):491–499
Rolnik DL, Wright D, Poon LC, O’gorman N, Syngelaki A, de PacoMatallana C, Molina FS, Nicolaides KH (2017) Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 377(7):613–622
Roberge S, Bujold E, Nicolaides KH (2018) Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol 218(3):287–293
Cadavid AP (2017) Aspirin: the mechanism of action revisited in the context of pregnancy complications. Front Immunol 8:261
Hui D, Okun N, Murphy K, Kingdom J, Uleryk E, Shah PS (2012) Combinations of maternal serum markers to predict preeclampsia, small for gestational age, and stillbirth: a systematic review. J Obstet Gynaecol Can 34(2):142–153
Clifton VL, Stark MJ, Osei-Kumah A, Hodyl NA (2012) The feto-placental unit, pregnancy pathology and impact on long term maternal health. Placenta 33:S37–S41
Thorp JA, Walsh SW, Brath PC (1988) Low-dose aspirin inhibits thromboxane, but not prostacyclin, production by human placental arteries. Am J Obstet Gynecol 159(6):1381–1384
Chakraborty C, Gleeson LM, McKinnon T, Lala PK (2002) Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol 80(2):116–124
Bose P, Black S, Kadyrov M, Weissenborn U, Neulen J, Regan L, Huppertz B (2005) Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am J Obstet Gynecol 192(1):23–30
Han CS, Mulla MJ, Brosens JJ, Chamley LW, Paidas MJ, Lockwood CJ, Abrahams VM (2011) Aspirin and heparin effect on basal and antiphospholipid antibody modulation of trophoblast function. Obstet Gynecol 118(5):1021–1028
Panagodage S, Yong HE, Costa FDS, Borg AJ, Kalionis B, Brennecke SP, Murthi P (2016) Low-dose acetylsalicylic acid treatment modulates the production of cytokines and improves trophoblast function in an in vitro model of early-onset preeclampsia. Am J Pathol 186(12):3217–3224
Kusuma GD, Manuelpillai U, Abumaree MH, Pertile MD, Brennecke SP, Kalionis B (2015) Mesenchymal stem cells reside in a vascular niche in the decidua basalis and are absent in remodelled spiral arterioles. Placenta 36(3):312–321
da Silva Meirelles L, Chagastelles PC, Nardi NB (2006) Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119(11):2204–2213
Kusuma GD, Abumaree MH, Pertile MD, Perkins AV, Brennecke SP, Kalionis B (2016) Mesenchymal stem/stromal cells derived from a reproductive tissue niche under oxidative stress have high aldehyde dehydrogenase activity. Stem Cell Rev Rep 12(3):285–297
Hwang JH, Lee MJ, Seok OS, Paek YC, Cho GJ, Seol HJ, Oh MJ (2010) Cytokine expression in placenta-derived mesenchymal stem cells in patients with pre-eclampsia and normal pregnancies. Cytokine 49(1):95–101
Rolfo A, Giuffrida D, Nuzzo AM, Pierobon D, Cardaropoli S, Piccoli E, Todros T (2013) Pro-inflammatory profile of preeclamptic placental mesenchymal stromal cells: new insights into the etiopathogenesis of preeclampsia. PLoS One 8(3):e59403
Leonhardt A, Bernert S, Watzer B, Schmitz-Ziegler G, Seyberth HW (2003) Low-dose aspirin in pregnancy: maternal and neonatal aspirin concentrations and neonatal prostanoid formation. Pediatrics 111(1):77–81
Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179(3):1855–1863
Lyer SS, Rojas M (2008) Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther 8(5):569–581
Fu L, Liu Y, Zhang D, Xie J, Guan H, Shang T (2015) Beneficial effect of human umbilical cord derived mesenchymal stem cells on an endotoxin induced rat model of preeclampsia. Experimental Therapeutic Medicine 10(5):1851–1856
Valle-Prieto A, Conget PA (2010) Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 19(12):1885–1893
Chen JW, Zhou SB, Tan ZM (2010) Aspirin and pravastatin reduce lectin-like oxidized low density lipoprotein receptor-1 expression, adhesion molecules and oxidative stress in human coronary artery endothelial cells. Chin Med J 123(12):1553–1556
Wu Y, Zhai H, Wang Y, Li L, Wu J, Wang F et al (2012) Aspirin-triggered lipoxin A4 attenuates lipopolysaccharide-induced intracellular ROS in BV2 microglia cells by inhibiting the function of NADPH oxidase. Neurochem Res 37(8):1690–1696
Zhang F, Lu M, Wang H, Ren T (2013) Aspirin attenuates angiotensin II induced inflammation in bone marrow mesenchymal stem cells via the inhibition of ERK1/2 and NF κB activation. Biomedical Reports 1(6):930–934
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25(11):2739–2749
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nature reviews. Immunology 8(9):726
Ehninger A, Trumpp A (2011) The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med 208(3):421–428
Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S et al (2011) Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 8(2):177–187
Alimperti S, Mirabella T, Bajaj V, Polacheck W, Pirone DM, Duffield J, Eyckmans J, Assoian RK, Chen CS (2017) Three-dimensional biomimetic vascular model reveals a RhoA, Rac1, and N-cadherin balance in mural cell–endothelial cell-regulated barrier function. Proc Natl Acad Sci 114(33):8758–8763
Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV (2007) Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 25(7):1761–1768
Liu L, Zhao G, Fan H, Zhao X, Li P, Wang Z et al (2014) Mesenchymal stem cells ameliorate Th1-induced pre-eclampsia-like symptoms in mice via the suppression of TNF-α expression. PLoS One 9(2):e88036
Tang J, Xiong J, Wu T, Tang Z, Ding G, Zhang C et al (2014) Aspirin treatment improved mesenchymal stem cell immunomodulatory properties via the 15d-PGJ2/PPARγ/TGF-β1 pathway. Stem Cells Dev 23(17):2093–2103
Acknowledgements
We acknowledge the patients who consented to provide their placenta, and the clinical research midwives at the Royal Women’s Hospital, Sue Duggan and Moira Stewart for sample collection.
Author information
Authors and Affiliations
Contributions
Ramin Khanabdali: Conception and design, collection and assembly of data, data analysis and interpretations, manuscript writing, and final approval of the manuscript.
Aida Shakouri-Motlagh: Conception and design, collection and assembly of data, data analysis and interpretations, and final approval of the manuscript.
Sarah Wilkinson: Collection and assembly of data and final approval of the manuscript.
Padma Murthi: Manuscript editing and final approval of the manuscript.
Harry M Georgiou: Manuscript editing and final approval of the manuscript.
Shaun P. Brennecke: Provision of study material or patients and final approval of the manuscript.
Bill Kalionis: Conception and design, data interpretations, manuscript writing, and final approval of the manuscript.
Corresponding author
Ethics declarations
The study was approved by the Human Research Ethics Committee of the Royal Women’s Hospital, Victoria, Australia.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 124 kb)
Rights and permissions
About this article
Cite this article
Khanabdali, R., Shakouri-Motlagh, A., Wilkinson, S. et al. Low-dose aspirin treatment enhances the adhesion of preeclamptic decidual mesenchymal stem/stromal cells and reduces their production of pro-inflammatory cytokines. J Mol Med 96, 1215–1225 (2018). https://doi.org/10.1007/s00109-018-1695-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-018-1695-9