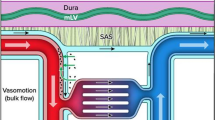
Abstract
The lymphatic vasculature act as the drainage system for most of our tissues and organs, clearing interstitial fluid and waste and returning them to the blood circulation. This is not the case for the central nervous system (CNS), which is devoid of parenchymal lymphatic vessels. Nevertheless, the brain is responsible for 25% of the body’s metabolism and only compromises 2% of the body’s mass. This high metabolic load requires an efficient system to remove waste products and maintain homeostasis. Well-described mechanisms of waste clearance include phagocytic immune cell functions as well as perivascular fluid flow; however, the need for active drainage of waste from the brain is becoming increasingly appreciated. Recent developments in lymphatic vascular biology challenge the proposition that the brain lacks lymphatic drainage or an equivalent. In this review, we describe the roles of the glymphatic system (a key drainage mechanism in the absence of lymphatics), the recently characterized meningeal lymphatic vessels, and explore an enigmatic cell population found in zebrafish called mural lymphatic endothelial cells. These systems may play important individual and collective roles in draining and clearing wastes from the brain.


Similar content being viewed by others
References
Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K (2016) Lymphatic system in cardiovascular medicine. Circ Res 118(3):515–530
Baruch K, Kertser A, Porat Z, Schwartz M (2015) Cerebral nitric oxide represses choroid plexus NFkappaB-dependent gateway activity for leukocyte trafficking. EMBO J 34(13):1816–1828
Prinz M, Erny D, Hagemeyer N (2017) Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol 18(4):385–392
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5(12):953–964
Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA (1985) Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326(1):47–63
Rennels ML, Blaumanis OR, Grady PA (1990) Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol 52:431–439
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4(147):147ra111. https://doi.org/10.1126/scitranslmed.3003748
Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M (2013) Cerebral arterial pulsation drives paravascular CSF—interstitial fluid exchange in the murine brain. J Neurosci 33(46):18190–18199
Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS (2017) Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6. https://doi.org/10.7554/eLife.27679
Jin BJ, Smith AJ, Verkman AS (2016) Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 148(6):489–501
Asgari M, de Zelicourt D, Kurtcuoglu V (2016) Glymphatic solute transport does not require bulk flow. Sci Rep 6:38635. https://doi.org/10.1038/srep38635
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123(3):1299–1309
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science (New York, NY) 342 (6156). https://doi.org/10.1126/science.1241224
Kress BT, Iliff JJ, Xia M, Wang M, Wei H, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew J, Plog BA, Ding F, Deane R, Nedergaard M (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76(6):845–861
Weed LH (1914) Studies on cerebro-spinal fluid. No. III : the pathways of escape from the subarachnoid spaces with particular reference to the arachnoid villi. J Med Res 31(1):51–91
Upton ML, Weller RO (1985) The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg 63(6):867–875
Go KG, Houthoff HJ, Hartsuiker J, Blaauw EH, Havinga P (1986) Fluid secretion in arachnoid cysts as a clue to cerebrospinal fluid absorption at the arachnoid granulation. J Neurosurg 65(5):642–648
Boulton M, Young A, Hay J, Armstrong D, Flessner M, Schwartz M, Johnston M (1996) Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125I-albumin clearance. Neuropathol Appl Neurobiol 22(4):325–333
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D (2004) Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1(1):2
Kida S, Pantazis A, Weller RO (1993) CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19(6):480–488
Mascagni Pe (1787) De lymphaticis profundis capitis et colli. Vasorum lymphaticorum corporis humani historia et ichnographia. Pars Prima section Siena: Pazzini Carli VII, Art. VI
Lecco V (1953) Di una probabile modificazione delle fissure linfatiche della della parte dei seni venosi della dura madre. Arch Ital Otol Rinol Laringol 64:287–296
Li J, Zhou J, Shi Y (1996) Scanning electron microscopy of human cerebral meningeal stomata. Ann Anat = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft 178(3):259–261
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341
Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204(10):2349–2362
Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS (2017) Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6. https://doi.org/10.7554/eLife.29738
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212(7):991–999
Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438(7070):946–953
Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, Fang S, Aspelund A, Saarma M, Eichmann A, Thomas JL, Alitalo K (2017) Development and plasticity of meningeal lymphatic vessels. J Exp Med 214(12):3645–3667. https://doi.org/10.1084/jem.20170391
Ma Q, Ineichen BV, Detmar M, Proulx ST (2017) Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 8(1):1434. https://doi.org/10.1038/s41467-017-01484-6
Raper D, Louveau A, Kipnis J (2016) How do meningeal lymphatic vessels drain the CNS? Trends Neurosci 39(9):581–586
Bower NI, Koltowska K, Pichol-Thievend C, Virshup I, Paterson S, Lagendijk AK, Wang W, Lindsey BW, Bent SJ, Baek S, Rondon-Galeano M, Hurley DG, Mochizuki N, Simons C, Francois M, Wells CA, Kaslin J, Hogan BM (2017) Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. NatNeurosci 20(6):774–783. https://doi.org/10.1038/nn.4558
Venero Galanternik M, Castranova D, Gore AV, Blewett NH, Jung HM, Stratman AN, Kirby MR, Iben J, Miller MF, Kawakami K (2017) A novel perivascular cell population in the zebrafish brain. ELife 6. https://doi.org/10.7554/eLife.24369
van Lessen M, Shibata-Germanos S (2017) Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. ELife 6. https://doi.org/10.7554/eLife.25932
Mato M, Ookawara S (1981) Influences of age and vasopressin on the uptake capacity of fluorescent granular perithelial cells (FGP) of small cerebral vessels of the rat. Am J Anat 162(1):45–53
Mato M, Ookawara S, Sano M, Kurihara K (1982) Studies on cerebral scavenger cells (fluorescent granular perithelial cells)—especially uptake and digestion of incorporated fat. No to shinkei = Brain and nerve 34(10):989–997
Mato M, Ookawara S, Sano M, Fukuda S (1982) Uptake of fat by fluorescent granular perithelial cells in cerebral cortex after administration of fat rich chow. Experientia 38(12):1496–1498
Mato M, Ookawara S, Sakamoto A, Aikawa E, Ogawa T, Mitsuhashi U, Masuzawa T, Suzuki H, Honda M, Yazaki Y, Watanabe E, Luoma J, Yla-Herttuala S, Fraser I, Gordon S, Kodama T (1996) Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc Natl Acad Sci U S A 93(8):3269–3274
Mato M, Ookawara S, Sugamata M, Aikawa E (1984) Evidence for the possible function of the fluorescent granular perithelial cells in brain as scavengers of high-molecular-weight waste products. Experientia 40(4):399–402
Shimabukuro MK, Langhi LG, Cordeiro I, Brito JM, Batista CM, Mattson MP, Mello Coelho V (2016) Lipid-laden cells differentially distributed in the aging brain are functionally active and correspond to distinct phenotypes. Sci Rep 6:23795. https://doi.org/10.1038/srep23795
Kida S, Steart PV, Zhang ET, Weller RO (1993) Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol 85(6):646–652
Katsumoto A, Lu H, Miranda AS, Ransohoff RM (2014) Ontogeny and functions of central nervous system macrophages. J Immunol (Baltimore, Md : 1950) 193(6):2615–2621
Balabanov R, Washington R, Wagnerova J, Dore-Duffy P (1996) CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res 52(2):127–142
Perry VH, Teeling J (2013) Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol 35(5):601–612
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Menard J, Zetterberg H, Wisniewski T, de Leon MJ (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11(8):457–470
Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J (2017) Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127(9):3210–3219
Jessen NA, Munk AS, Lundgaard I, Nedergaard M (2015) The glymphatic system: a beginner’s guide. Neurochem Res 40(12):2583–2599
Louveau A, Da Mesquita S, Kipnis J (2016) Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron 91(5):957–973
Funding
B.M.H. by an NHMRC/National Heart Foundation Career Development Fellowship (1083811). N.I.B. was supported in part by NHMRC Project grant (1079670).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bower, N.I., Hogan, B.M. Brain drains: new insights into brain clearance pathways from lymphatic biology. J Mol Med 96, 383–390 (2018). https://doi.org/10.1007/s00109-018-1634-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-018-1634-9