Abstract
Purpose
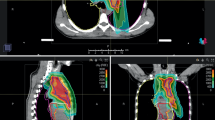
Current research approaches in lymphoma focus on reduction of therapy-associated long-term side effects. Especially in mediastinal lymphoma, proton beam radiotherapy (PT) may be a promising approach for reducing the dose to organs at risk (OAR).
Patients
In total, 20 patients were irradiated with active scanning PT at Heidelberg Ion Beam Therapy Center (HIT) between September 2014 and February 2017. For comparative analysis, additional photon irradiation plans with helical intensity-modulated radiotherapy (IMRT) were calculated and quantitative and qualitative dose evaluations were made for both treatment modalities. Toxicity and survival outcomes were evaluated.
Results
Clinical target volume coverage was comparable in both treatment modalities and did not significantly differ between IMRT and PT. Nevertheless, PT showed superiority regarding the homogeneity index (HIPT = 1.041 vs. HIIMRT = 1.075, p < 0.001). For all OAR, PT showed significantly higher dose reductions compared with IMRT. In particular, the dose to the heart was reduced in PT (absolute dose reduction of Dmean of 3.3 Gy [all patients] and 4.2 Gy [patients with pericardial involvement]). Likewise, the subgroup analysis of female patients, who were expected to receive higher doses to the breast, showed a higher dose reduction in Dmean of 1.2 Gy (right side) and 2.2 Gy (left side). After a median follow-up of 32 months (range 21–48 months), local and distant progression free survival (LPFS and DPFS) were 95.5% and 95.0%, respectively. Radiotherapy was tolerated well with only mild (grade 1–2) radiation-induced acute and chronic side effects.
Conclusion
A significant reduction in the dose to the surrounding OAR was achieved with PT compared with photon irradiation, without compromising target volume coverage. Dosimetric advantages may have the potential to translate into a reduction of long-term radiation-induced toxicity in young patients with malignant lymphoma of the mediastinum.
Zusammenfassung
Zielsetzung
Forschungsschwerpunkte in der Therapie von Lymphompatienten befassen sich zunehmend mit der Reduktion von Spättoxizitäten. Aufgrund der verbesserten Dosisreduktion an Risikoorganen (OAR) kann die Protonenradiotherapie (PT), insbesondere bei Patienten mit mediastinalen Lymphomen, einen vielversprechenden Therapieansatz darstellen.
Methoden und Patienten
Von September 2014 bis Februar 2017 behandelten wir 20 Patienten (medianes Alter 31 Jahre) mittels aktivem Rasterscanning mit einer PT. Zusätzliche Photonenbestrahlungspläne mittels helikaler intensitätsmodulierter Radiotherapie (IMRT) wurden berechnet und quantitative und qualitative Vergleichsanalysen durchgeführt. Zudem wurden Toxizitäten und Ansprechraten evaluiert.
Ergebnisse
Die klinische Zielvolumenabdeckung war vergleichbar und zeigte keine signifikanten Unterschiede zwischen beiden Behandlungsmodalitäten. Es zeigte sich jedoch eine Überlegenheit der PT bezüglich der Homogenität (Homogenitätsindex [HI] HIPT = 1,041 vs. HIIMRT = 1,075; p < 0,001). Des Weiteren ließ sich mittels PT eine signifikante Dosisreduktion an den OAR erreichen. Insbesondere die mittlere Herzdosis zeigte eine signifikante absolute Dosisreduktion von Dmean 3,3 Gy (bei allen Patienten) und 4,2 Gy (bei Patienten mit präkardialem Lymphombefall). Zudem profitierten Patientinnen von einer Dosisreduktion am Brustdrüsengewebe mit einer absoluten Reduktion von Dmean 1,2 Gy (rechts) und 2,2 Gy (links). Nach einem medianen Follow-up von 32 Monaten (Spanne 21–48 Monate) betrug das lokale bzw. distante progressionsfreie Überleben (LPFS bzw. DPFS) 95,5 % bzw. 95,0 %. Es traten lediglich milde Nebenwirkungen (Grad 1–2) durch die Bestrahlung auf.
Schlussfolgerung
Mittels PT zeigt sich im Vergleich zu einer Photonenbestrahlung eine signifikante Dosisreduktion angrenzender OAR bei Patienten mit mediastinalen Lymphomen, ohne dabei eine Beeinträchtigung der Zielvolumenabdeckung in Kauf zu nehmen. Diese dosimetrischen Vorteile bergen insbesondere bei jungen Patienten mit mediastinalen Lymphomen das Potenzial, sich in einer Reduktion der Langzeittoxizitäten niederzuschlagen.


Similar content being viewed by others
References
Girinsky T et al (2006) Is intensity-modulated radiotherapy better than conventional radiation treatment and three-dimensional conformal radiotherapy for mediastinal masses in patients with Hodgkin’s disease, and is there a role for beam orientation optimization and dose constraints assigned to virtual volumes? Int J Radiat Oncol Biol Phys 64(1):218–226
Goodman KA et al (2005) Intensity-modulated radiotherapy for lymphoma involving the mediastinum. Int J Radiat Oncol Biol Phys 62(1):198–206
Held G et al (2014) Role of radiotherapy to bulky disease in elderly patients with aggressive B‑cell lymphoma. J Clin Oncol 32(11):1112–1118
Ng AK et al (2016) Re-examining the role of radiation therapy for diffuse large B‑cell lymphoma in the modern era. J Clin Oncol 34(13):1443–1447
Pfreundschuh M et al (2011) CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6‑year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 12(11):1013–1022
Engert A et al (2010) Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med 363(7):640–652
von Tresckow B et al (2012) Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol 30(9):907–913
Engert A et al (2012) Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet 379(9828):1791–1799
Illidge T et al (2014) Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 89(1):49–58
Specht L et al (2014) Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys 89(4):854–862
Kramer M et al (2000) Treatment planning for heavy-ion radiotherapy: physical beam model and dose optimization. Phys Med Biol 45(11):3299–3317
Kramer M et al (2004) Treatment planning for scanned ion beams. Radiother Oncol 73(Suppl 2):S80–S85
Kramer M, Scholz M (2000) Treatment planning for heavy-ion radiotherapy: calculation and optimization of biologically effective dose. Phys Med Biol 45(11):3319–3330
Voong KR et al (2014) Dosimetric advantages of a “butterfly” technique for intensity-modulated radiation therapy for young female patients with mediastinal Hodgkin’s lymphoma. Radiat Oncol 9:94
Emami B et al (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21(1):109–122
Haberer T et al (1993) Magnetic scanning system for heavy ion therapy. Nucl Instrum Methods Phys Res A 330(1):296–305
Kataria T et al (2012) Homogeneity index: an objective tool for assessment of conformal radiation treatments. J Med Phys 37(4):207–213
Shaw E et al (1993) Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 27(5):1231–1239
Kessel KA et al (2014) Five-year experience with setup and implementation of an integrated database system for clinical documentation and research. Comput Methods Programs Biomed 114(2):206–217
Bougatf N, Bendl R, Debus J (2015) Towards secondary use of heterogeneous radio-oncological data for retrospective clinical trials: service-oriented connection of a central research database with image analysis tools
Cheson BD et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Hoppe BS et al (2012) Consolidative involved-node proton therapy for Stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a Phase II study. Int J Radiat Oncol Biol Phys 83(1):260–267
Hoppe BS et al (2012) Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 84(2):449–455
Zeng C et al (2016) Proton pencil beam scanning for mediastinal lymphoma: treatment planning and robustness assessment. Acta Oncol 55(9–10):1132–1138
Oeffinger KC et al (2006) Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355(15):1572–1582
Held G et al (2013) Impact of rituximab and radiotherapy on outcome of patients with aggressive B‑cell lymphoma and skeletal involvement. J Clin Oncol 31(32):4115–4122
Borchmann P et al (2011) Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol 29(32):4234–4242
Girinsky T et al (2008) The conundrum of Hodgkin lymphoma nodes: to be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol 88(2):202–210
Holzhauser E et al (2017) Patterns of failure of diffuse large Bcell lymphoma patients after involved-site radiotherapy. Strahlenther Onkol 193(12):1014–1023
Maraldo MV et al (2014) The impact of involved node, involved field and mantle field radiotherapy on estimated radiation doses and risk of late effects for pediatric patients with Hodgkin lymphoma. Pediatr Blood Cancer 61(4):717–722
Hall EJ (2006) Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys 65(1):1–7
van Nimwegen FA et al (2016) Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin Lymphoma. J Clin Oncol 34(3):235–243
Lipshultz SE et al (2013) Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 128(17):1927–1995
Tukenova M et al (2010) Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 28(8):1308–1315
van Leeuwen FE, Ng AK (2016) Long-term risk of second malignancy and cardiovascular disease after Hodgkin lymphoma treatment. Hematology Am Soc Hematol Educ Program 2016(1):323–330
van Nimwegen FA et al (2017) Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 129(16):2257–2265. https://doi.org/10.1182/blood-2016-09-740332
Mulrooney DA et al (2016) Cardiac outcomes in adult survivors of childhood cancer exposed to Cardiotoxic therapy: a cross-sectional study. Ann Intern Med 164(2):93–101
Schaapveld M et al (2015) Second cancer risk up to 40 years after treatment for Hodgkin’s Lymphoma. N Engl J Med 373(26):2499–2511
Moskowitz CS et al (2014) Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol 32(21):2217–2223
De Bruin ML et al (2009) Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 27(26):4239–4246
Jiang HY et al (2005) Simulation of organ-specific patient effective dose due to secondary neutrons in proton radiation treatment. Phys Med Biol 50(18):4337–4353
Andolino DL et al (2011) Dosimetric comparison of involved-field three-dimensional conformal photon radiotherapy and breast-sparing proton therapy for the treatment of Hodgkin’s lymphoma in female pediatric patients. Int J Radiat Oncol Biol Phys 81(4):e667–71
Schneider U et al (2002) Secondary neutron dose during proton therapy using spot scanning. Int J Radiat Oncol Biol Phys 53(1):244–251
Schneider U, Lomax A, Lombriser N (2000) Comparative risk assessment of secondary cancer incidence after treatment of Hodgkin’s disease with photon and proton radiation. Radiat Res 154(4):382–388
Chung CS et al (2013) Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys 87(1):46–52
Halg RA et al (2014) Measurements of the neutron dose equivalent for various radiation qualities, treatment machines and delivery techniques in radiation therapy. Phys Med Biol 59(10):2457–2468
Schneider U, Halg R (2015) The impact of neutrons in clinical proton therapy. Front Oncol 5:235
Gottschalk B (2006) Neutron dose in scattered and scanned proton beams: in regard to Eric J. Hall (Int J Radiat Oncol Biol Phys 2006;65:1–7). Int J Radiat Oncol Biol Phys 66(5):1594 (author reply 1595)
Richter D et al (2014) Four-dimensional patient dose reconstruction for scanned ion beam therapy of moving liver tumors. Int J Radiat Oncol Biol Phys 89(1):175–181
Acknowledgements
This work was supported by the Medical Faculty of Heidelberg providing a research grant for LK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. König, N. Bougatf, J. Hörner-Rieber, N. Chaudhri, T. Mielke, S. Klüter, M.F. Haefner, S. Rieken, T. Haberer, J. Debus and K. Herfarth declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from the local Ethics Committee of Heidelberg University (S-201/2017).
Rights and permissions
About this article
Cite this article
König, L., Bougatf, N., Hörner-Rieber, J. et al. Consolidative mediastinal irradiation of malignant lymphoma using active scanning proton beams: clinical outcome and dosimetric comparison. Strahlenther Onkol 195, 677–687 (2019). https://doi.org/10.1007/s00066-019-01460-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01460-7
Keywords
- Mediastinal lymphoma
- Intensity modulated radiotherapy
- Secondary malignancies
- Proton therapy
- Radiotherapy
- Toxicity