Abstract
Background
Radiotherapy (RT) has been known for decades as a local treatment modality for malign and benign disease. In order to efficiently exploit the therapeutic potential of RT, an understanding of the immune modulatory properties of ionizing radiation is mandatory. These should be used for improvement of radioimmunotherapies for cancer in particular.
Methods
We here summarize the latest research and review articles about immune modulatory properties of RT, with focus on radiation dose and on combination of RT with selected immunotherapies. Based on the knowledge of the manifold immune mechanisms that are triggered by RT, thought-provoking impulse for multimodal radioimmunotherapies is provided.
Results
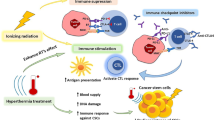
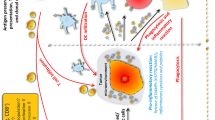
It has become obvious that ionizing radiation induces various forms of cell death and associated processes via DNA damage initiation and triggering of cellular stress responses. Immunogenic cell death (ICD) is of special interest since it activates the immune system via release of danger signals and via direct activation of immune cells. While RT with higher single doses in particular induces ICD, RT with a lower dose is mainly responsible for immune cell recruitment and for attenuation of an existing inflammation. The counteracting immunosuppression emanating from tumor cells can be overcome by combining RT with selected immunotherapies such as immune checkpoint inhibition, TGF-β inhibitors, and boosting of immunity with vaccination.
Conclusion
In order to exploit the full power of RT and thereby develop efficient radioimmunotherapies, the dose per fraction used in RT protocols, the fractionation, the quality, and the quantity of certain immunotherapies need to be qualitatively and chronologically well-matched to the individual immune status of the patient.
Zusammenfassung
Hintergrund
Strahlentherapie (ST) wird seit Jahrzehnten für die lokale Behandlung von benignen und malignen Krankheitsbildern verwendet. Um das weitreichende Potenzial der ST effizienter zu nutzen, ist ein besseres Verständnis der immunmodulierenden Eigenschaften der ionisierenden Strahlung essenziell. Dieses Wissen sollte auch für die Verbesserung von Strahlenimmuntherapien gegen Krebs verwendet werden.
Methoden
Wir fassen die neuste Literatur über die immunmodulierenden Eigenschaften der ST im Bezug zu einer bestimmten Dosis und in Kombination mit Immuntherapien zusammen. Basierend auf diesem Wissen über die vielfältigen immunologischen Mechanismen, die durch ST hervorgerufen werden, diskutieren wir neue multimodale Strahlenimmuntherapieansätze.
Ergebnisse
Es zeigte sich, dass ionisierende Strahlung verschiedene Zelltodesformen und assoziierte Prozesse als Folge von DNA-Schadens- und zellulären Stressantworten hervorruft. Dabei ist der immunogene Zelltod (IZT) von besonderem Interesse, da er das Immunsystem indirekt durch Freisetzung von Gefahrensignalen und durch direkte Aktivierung von Immunzellen aktiviert. Während ST mit höheren Einzeldosen pro Fraktion besonders IZT induziert, ist ST mit niedrigeren Dosen pro Fraktion hauptsächlich für die Rekrutierung von Immunzellen und die Abschwächung von vorherrschenden Entzündungen verantwortlich. Einer von den Tumorzellen ausgehenden Immunsuppression kann mit einer Kombination von ST und darauf angepassten Immuntherapien, wie z. B. Checkpoint-Inhibitoren, TGF-β-Inhibitoren oder Vakzinierung entgegengewirkt werden.
Schlussfolgerung
Um das volle Potenzial der ST auszuschöpfen, müssen Strahlenimmuntherapien entwickelt werden, bei denen verwendete ST-Einzeldosen, Fraktionierung sowie Qualität und Quantität bestimmter Immuntherapien qualitativ und chronologisch genau auf den individuellen Immunstatus des Patienten abgestimmt sind.



Similar content being viewed by others
References
Wu Q, Allouch A, Martins I et al (2017) Modulating both tumor cell death and innate immunity is essential for improving radiation therapy effectiveness. Front Immunol 8:613
Lauber K, Ernst A, Orth M et al (2012) Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol 2:116
Castedo M, Perfettini JL, Roumier T et al (2004) Cell death by mitotic catastrophe: a molecular definition. Oncogene 23:2825–2837
Deloch L, Derer A, Hartmann J et al (2016) Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol 6:141
Willems JJ, Arnold BP, Gregory CD (2014) Sinister self-sacrifice: the contribution of apoptosis to malignancy. Front Immunol 5:299
Frey B, Schildkopf P, Rodel F et al (2009) AnnexinA5 renders dead tumor cells immunogenic—implications for multimodal cancer therapies. J Immunotoxicol 6:209–216
Werthmoller N, Frey B, Wunderlich R et al (2015) Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T‑cell-dependent manner. Cell Death Dis 6:e1761
Kaczmarek A, Vandenabeele P, Krysko DV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38:209–223
Muth C, Rubner Y, Semrau S et al (2016) Primary glioblastoma multiforme tumors and recurrence: comparative analysis of the danger signals HMGB1, HSP70, and calreticulin. Strahlenther Onkol 192:146–155
Lu C, Xie C (2016) Radiation-induced autophagy promotes esophageal squamous cell carcinoma cell survival via the LKB1 pathway. Oncol Rep 35:3559–3565
Chiu HW, Lin SW, Lin LC et al (2015) Synergistic antitumor effects of radiation and proteasome inhibitor treatment in pancreatic cancer through the induction of autophagy and the downregulation of TRAF6. Cancer Lett 365:229–239
Citrin DE (2017) Recent developments in radiotherapy. N Engl J Med 377:1065–1075
Kepp O, Senovilla L, Vitale I et al (2014) Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 3:e955691
Matzinger P (2002) The danger model: a renewed sense of self. Science 296:301–305
Gaipl US, Multhoff G, Scheithauer H et al (2014) Kill and spread the word: stimulation of antitumor immune responses in the context of radiotherapy. Immunotherapy 6:597–610
Shevtsov M, Multhoff G (2016) Heat shock protein-peptide and HSP-based immunotherapies for the treatment of cancer. Front Immunol 7:171
Stangl S, Tontcheva N, Sievert W et al (2017) Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: a multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int J Cancer. https://doi.org/10.1002/ijc.31213
Specht HM, Ahrens N, Blankenstein C et al (2015) Heat Shock Protein 70 (Hsp70) peptide activated Natural Killer (NK) cells for the treatment of patients with Non-Small Cell Lung Cancer (NSCLC) after Radiochemotherapy (RCTx)—from preclinical studies to a clinical phase II trial. Front Immunol 6:162
Garg AD, Galluzzi L, Apetoh L et al (2015) Molecular and translational classifications of DAMPs in immunogenic cell death. Front Immunol 6:588
Reits EA, Hodge JW, Herberts CA et al (2006) Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203:1259–1271
Frey B, Rubner Y, Kulzer L et al (2014) Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother 63:29–36
Galluzzi L, Buque A, Kepp O et al (2017) Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17:97–111
Wennerberg E, Lhuillier C, Vanpouille-Box C et al (2017) Barriers to radiation-induced in situ tumor vaccination. Front Immunol 8:229
Bouquet F, Pal A, Pilones KA et al (2011) TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res 17:6754–6765
Deng L, Liang H, Burnette B et al (2014) Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–695
Jobling MF, Mott JD, Finnegan MT et al (2006) Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res 166:839–848
Vanpouille-Box C, Diamond JM, Pilones KA et al (2015) TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res 75:2232–2242
Vanpouille-Box C, Alard A, Aryankalayil MJ et al (2017) DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 8:15618
Kang J, Demaria S, Formenti S (2016) Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 4:51
Ni L, Dong C (2017) New checkpoints in cancer immunotherapy. Immunol Rev 276:52–65
Dong H, Strome SE, Salomao DR et al (2002) Tumor-associated B7-H1 promotes T‑cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Derer A, Spiljar M, Baumler M et al (2016) Chemoradiation increases PD-L1 expression in certain melanoma and glioblastoma cells. Front Immunol 7:610
Dovedi SJ, Adlard AL, Lipowska-Bhalla G et al (2014) Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74:5458–5468
Twyman-Saint Victor C, Rech AJ, Maity A et al (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373–377
Derer A, Deloch L, Rubner Y et al (2015) Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses—pre-clinical evidence and ongoing clinical applications. Front Immunol 6:505
Golden EB, Chhabra A, Chachoua A et al (2015) Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 16:795–803
Frey B, Gaipl US (2015) Radio-immunotherapy: the focused beam expands. Lancet Oncol 16:742–743
Rodel F, Fournier C, Wiedemann J et al (2017) Basics of radiation biology when treating hyperproliferative benign diseases. Front Immunol 8:519
Frey B, Hehlgans S, Rodel F et al (2015) Modulation of inflammation by low and high doses of ionizing radiation: implications for benign and malign diseases. Cancer Lett 368:230–237
Rodel F, Frey B, Gaipl U et al (2012) Modulation of inflammatory immune reactions by low-dose ionizing radiation: molecular mechanisms and clinical application. Curr Med Chem 19:1741–1750
Wunderlich R, Ernst A, Rodel F et al (2015) Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol 179:50–61
Large M, Hehlgans S, Reichert S et al (2015) Study of the anti-inflammatory effects of low-dose radiation: the contribution of biphasic regulation of the antioxidative system in endothelial cells. Strahlenther Onkol 191:742–749
Rodel F, Hofmann D, Auer J et al (2008) The anti-inflammatory effect of low-dose radiation therapy involves a diminished CCL20 chemokine expression and granulocyte/endothelial cell adhesion. Strahlenther Onkol 184:41–47
Lodermann B, Wunderlich R, Frey S et al (2012) Low dose ionising radiation leads to a NF-kappaB dependent decreased secretion of active IL-1beta by activated macrophages with a discontinuous dose-dependency. Int J Radiat Biol 88:727–734
Ott OJ, Jeremias C, Gaipl US et al (2015) Radiotherapy for benign achillodynia. Long-term results of the Erlangen Dose Optimization Trial. Strahlenther Onkol 191:979–984
Ott OJ, Jeremias C, Gaipl US et al (2014) Radiotherapy for benign calcaneodynia: long-term results of the Erlangen Dose Optimization (EDO) trial. Strahlenther Onkol 190:671–675
Ott OJ, Hertel S, Gaipl US et al (2014) The Erlangen Dose Optimization Trial for radiotherapy of benign painful shoulder syndrome. Long-term results. Strahlenther Onkol 190:394–398
Ott OJ, Hertel S, Gaipl US et al (2014) The Erlangen Dose Optimization trial for low-dose radiotherapy of benign painful elbow syndrome. Long-term results. Strahlenther Onkol 190:293–297
Ruhle PF, Wunderlich R, Deloch L et al (2017) Modulation of the peripheral immune system after low-dose radon spa therapy: detailed longitudinal immune monitoring of patients within the RAD-ON01 study. Autoimmunity 50:133–140
Cucu A, Shreder K, Kraft D et al (2017) Decrease of markers related to bone erosion in serum of patients with musculoskeletal disorders after serial low-dose radon spa therapy. Front Immunol 8:882
Asur R, Butterworth KT, Penagaricano JA et al (2015) High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett 356:52–57
Herskind C, Ma L, Liu Q et al (2017) Biology of high single doses of IORT: RBE, 5 R’s, and other biological aspects. Radiat Oncol 12:24
Ghita M, Coffey CB, Butterworth KT et al (2016) Impact of fractionation on out-of-field survival and DNA damage responses following exposure to intensity modulated radiation fields. Phys Med Biol 61:515–526
Burnette BC, Liang H, Lee Y et al (2011) The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res 71:2488–2496
Demaria O, De Gassart A, Coso S et al (2015) STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci USA 112:15408–15413
Derer A, Frey B, Fietkau R et al (2016) Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunother 65:779–786
Klug F, Prakash H, Huber PE et al (2013) Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24:589–602
Frey B, Rubner Y, Wunderlich R et al (2012) Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation—implications for cancer therapies. Curr Med Chem 19:1751–1764
Vanpouille-Box C, Pilones KA, Wennerberg E et al (2015) In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 33:7415–7422
Frey B, Rückert M, Deloch L et al (2017) Immunomodulation by ionizing radiation—impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol Rev 280(1):231–248. https://doi.org/10.1111/imr.12572
Sahin U, Derhovanessian E, Miller M et al (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547:222–226
Yarchoan M, Johnson BA 3rd, Lutz ER et al (2017) Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17:569
Weiss EM, Wunderlich R, Ebel N et al (2012) Selected anti-tumor vaccines merit a place in multimodal tumor therapies. Front Oncol 2:132
Liu R, Luo F, Liu X et al (2016) Biological response modifier in cancer immunotherapy. Adv Exp Med Biol 909:69–138
Eckert F, Jelas I, Oehme M et al (2017) Tumor-targeted IL-12 combined with local irradiation leads to systemic tumor control via abscopal effects in vivo. Oncoimmunology 6:e1323161
Yang QY, Yang JD, Wang YS (2017) Current strategies to improve the safety of chimeric antigen receptor (CAR) modified T cells. Immunol Lett 190:201–205
Fournier C, Martin F, Zitvogel L et al (2017) Trial watch: adoptively transferred cells for anticancer immunotherapy. Oncoimmunology 6:e1363139
van der Burg SH, Arens R, Ossendorp F et al (2016) Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 16:219–233
Loi M, Desideri I, Greto D et al (2017) Radiotherapy in the age of cancer immunology: current concepts and future developments. Crit Rev Oncol Hematol 112:1–10
Harjes U (2017) Tumour vaccines: personal training by vaccination. Nat Rev Cancer 17:451–451
Muenst S, Soysal SD, Tzankov A et al (2015) The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Targets 19:201–211
Le DT, Durham JN, Smith KN et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409–413
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Topalian SL, Taube JM, Anders RA et al (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275–287
Weichselbaum RR, Liang H, Deng L et al (2017) Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol 14(6):365–379. https://doi.org/10.1038/nrclinonc.2016.211
Abuodeh Y, Venkat P, Kim S (2016) Systematic review of case reports on the abscopal effect. Curr Probl Cancer 40:25–37
Demaria S, Ng B, Devitt ML et al (2004) Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 58:862–870
Zheng W, Skowron KB, Namm JP et al (2016) Combination of radiotherapy and vaccination overcomes checkpoint blockade resistance. Oncotarget 7:43039–43051
Dewan MZ, Galloway AE, Kawashima N et al (2009) Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15:5379–5388
Frey B, Rückert M, Weber J et al (2017) Hypofractionated irradiation has immune stimulatory potential and induces a timely restricted infiltration of immune cells in colon cancer tumors. Front Immunol 8:231
Hettich M, Lahoti J, Prasad S, Niedermann G (2016) Checkpoint Antibodies but not T Cell-Recruiting Diabodies Effectively Synergize with TIL-Inducing γ-Irradiation. Cancer Res 76:4673–4683
Belka C, Ottinger H, Kreuzfelder E et al (1999) Impact of localized radiotherapy on blood immune cells counts and function in humans. Radiother Oncol 50:199–204
Heylmann D, Rodel F, Kindler T et al (2014) Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta 1846:121–129
Sage EK, Schmid TE, Geinitz H et al (2017) Effects of definitive and salvage radiotherapy on the distribution of lymphocyte subpopulations in prostate cancer patients. Strahlenther Onkol 193:648–655
van Meir H, Nout RA, Welters MJ et al (2017) Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 6:e1267095
Frey B, Ruckert M, Deloch L et al (2017) Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol Rev 280:231–248
Ruhle PF, Goerig N, Wunderlich R et al (2017) Modulations in the peripheral immune system of glioblastoma patient is connected to therapy and tumor progression-A case report from the IMMO-GLIO-01 trial. Front Neurol 8:296
Rühle PF, Fietkau R, Gaipl US et al (2016) Development of a modular assay for detailed immunophenotyping of peripheral human whole blood samples by multicolor flow cytometry. Int J Mol Sci 17(8):E1316. https://doi.org/10.3390/ijms17081316
Karakhanova S, Ryschich E, Mosl B et al (2015) Prognostic and predictive value of immunological parameters for chemoradioimmunotherapy in patients with pancreatic adenocarcinoma. Br J Cancer 112:1027–1036
Acknowledgements
This work was partially funded by the Bundesministerium für Bildung und Forschung (BMBF; GREWIS-alpha, 02NUK050E) and by the Research Training Group GRK1660 of the German Research Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Rückert, L. Deloch, R. Fietkau, B. Frey, M. Hecht, and U.S. Gaipl declare that they have no competing interests.
Additional information
Michael Rückert and Lisa Deloch contributed equally.
Rights and permissions
About this article
Cite this article
Rückert, M., Deloch, L., Fietkau, R. et al. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther Onkol 194, 509–519 (2018). https://doi.org/10.1007/s00066-018-1287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1287-1