Abstract
Background
To date, it remains unclear whether locally advanced adenocarcinoma of the gastroesophageal junction (AEG) should be treated with neoadjuvant chemoradiation (nCRT), analogous to esophageal cancer, or with perioperative chemotherapy (pCT), analogous to gastric cancer. The purpose of this study was to analyze the data of the Munich Cancer Registry (MCR) and to compare pCT and nCRT in AEG patients.
Patients and methods
A total of 2,992 AEG patients, treated between 1998 and 2014, were included in the study. Baseline and tumor parameters as well as overall survival (OS) and tumor recurrence were compared between 56 patients undergoing nCRT and 64 patients undergoing pCT with UICC stage II/III cancer. In addition, uni- and multivariate analyses using Cox regression models were performed to evaluate the effect of tumor characteristics and treatment regimens on OS.
Results
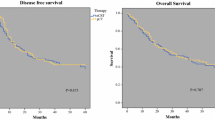
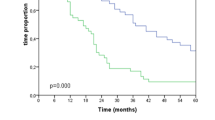
In patients with UICC stage II/III AEG treated with either nCRT or pCT, no significant differences were seen for baseline and tumor characteristics. While there was a significantly higher cumulative incidence of locoregional treatment failure after pCT (32.8%; 95% CI: 18.0–48.4%) compared with nCRT (7.4%; 95% CI: 2.3–16.5%; p = 0.007), there was no significant difference for distant treatment failure (52.9%; 95% CI: 35.4–67.7% and 38.4%; 95% CI: 23.7–52.9%; p = 0.347). When analyzing the whole cohort, patients who received pCT were younger (58.3 years vs. 63.0 years; p = 0.016), had a higher chance of complete tumor resection (81% vs. 67%; p = 0.033), more resected lymph nodes (p = 0.036), and fewer lymph node metastases (p = 0.038) compared with patients who received nCRT. Nevertheless, there was still a strong trend toward a higher incidence of local treatment failure after pCT (25.8%; 95% CI: 14.7–38.3% vs. 12.6%; 95% CI: 5.5–22.8%; p = 0.053). Comparable to the results for patients with UICC stage II/III, no difference was seen for the incidence of distant treatment failure. When excluding patients with UICC stage IV cancer, no significant difference was found for OS.
Conclusion
For UICC stage II/III carcinoma, nCRT was associated with an improved locoregional tumor control compared with pCT, while no further significant differences were seen between nCRT and pCT for UICC stage II/III AEG. Moreover, there was a strong trend toward improved locoregional tumor control after nCRT when analyzing all patients treated with nCRT or pCT, despite these patients having higher risk factors.
Zusammenfassung
Hintergrund
Bis heute ist nicht eindeutig geklärt, ob lokal fortgeschrittene Adenokarzinome des gastroösophagealen Übergangs (AEG) wie Ösophaguskarzinome mit neoadjuvanter Radiochemotherapie (nCRT) oder wie Magenkarzinome mit perioperativer Chemotherapie zu behandeln sind. In dieser Arbeit wird die Effektivität beider Verfahren anhand der Daten des Münchner Tumorregisters verglichen.
Material und Methoden
Ausgewertet wurden Daten von 2992, zwischen 1998 und 2014 behandelten, Patienten mit AEG. Patientencharakteristika, Tumorparameter, Gesamtüberleben (OS) und Rezidivraten von 56 (nCRT) bzw. 64 Patienten (pCT) mit AEG in den UICC-Stadien II und III wurden miteinander verglichen. Zusätzlich erfolgten uni- und multivariate Analysen mithilfe eines Cox-Regressions-Modells, um den Einfluss von Tumorcharakteristika und Behandlungsprotokollen auf das OS zu untersuchen.
Ergebnisse
Bei den AEG im UICC-Stadium II und III zeigten sich zwischen Patienten mit nCRT und Patienten mit pCT keine signifikanten Unterschiede bei Patientencharakteristika und Tumorparametern. Die kumulative Inzidenz von Lokalrezidiven war nach pCT (32,8 %; 95%-KI: 18,0–48,4 %) signifikant höher als nach nCRT (7,4 %; 95%-KI: 2,3–16,5 %) (p = 0,007). Für die kumulative Inzidenz der systemischen Rezidive ergab sich kein signifikanter Unterschied zwischen den Behandlungsgruppen (52,9 %; 95%-KI: 35,4–67,7 % bzw. 38,4 %; 95%-KI: 23,7–52,9 %) (p = 0,347). In der Gesamtkohorte waren Patienten mit pCT signifikant jünger (58,3 Jahre vs. 63,0 Jahre, p = 0,016), hatten häufiger eine komplette Tumorresektion (81 % vs. 67 %, p = 0,033), mehr entfernte Lymphnoten (p = 0,036) und weniger Lymphknotenmetastasen in den resezierten Lymphnoten (p = 0,038) als Patienten mit nCRT. Bezüglich der kumulativen Inzidenz der Lokalrezidive zeigte sich auch hier ein starker Trend hinsichtlich einer verbesserten Lokalkontrolle nach nCRT (25,8 %; 95%-KI: 14,7–38,3 % vs. 12,6 %; 95%-KI: 5,5–22,8 %; p = 0,053). Es ergab sich kein signifikanter Unterschied bezüglich der kumulativen Inzidenz der systemischen Rezidive. Nach Ausschluss von Patienten mit UICC-Stadium IV zeigte sich kein signifikanter Unterschied für das OS.
Schlussfolgerung
In der Subgruppe der AEG im UICC-Stadium II/III zeigte sich nach nCRT eine bessere lokale Kontrolle als nach pCT ohne signifikante Unterschiede bezüglich des OS oder der kumulativen Inzidenz der systemischen Rezidive. In der Gesamtkohorte zeigte sich trotz vermehrter Risikofaktoren zumindest ein starker Trend zu einer verbesserten lokalen Kontrolle nach nCRT.





Similar content being viewed by others
References
Ajani JA, D’Amico TA, Almhanna K et al (2015) Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 13(2):194–227
Al-Batran SE, Hofheinz RD, Pauligk C et al (2016) Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 17(12):1697–1708
Allum WH, Stenning SP, Bancewicz J et al (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27(30):5062–5067
Burmeister BH, Smithers BM, Gebski V et al (2005) Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 6(9):659–668
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355(1):11–20
D’Agostino RB Jr., D’Agostino RB Sr. (2007) Estimating treatment effects using observational data. JAMA 297(3):314–316
Hall MD, Schultheiss TE, Smith DD et al (2015) Impact of total lymph node count on staging and survival after neoadjuvant chemoradiation therapy for rectal cancer. Ann Surg Oncol 22(Suppl 3):S580–S587
Holscher AH, Stahl M, Messmann H et al (2016) New S3 guideline for esophageal cancer : Important surgical aspects. Chirurg 87(10):865–872
Hulshof MCCM, Oppedijk V, van der Gaast A (2014) Reply to E.C. Smyth et al. J Clin Oncol 32(27):3081–3082
Kelsen DP, Winter KA, Gunderson LL et al (2007) Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 25(24):3719–3725
Kim HJ, Jo JS, Lee SY et al (2015) Low lymph node retrieval after preoperative chemoradiation for rectal cancer is associated with improved prognosis in patients with a good tumor response. Ann Surg Oncol 22(6):2075–2081
Klevebro F, Alexandersson von Dobeln G, Wang N et al (2016) A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 27(4):660–667
Medical Research Council Oesophageal Cancer Working Party (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359(9319):1727–1733
Miccio JA, Oladeru OT, Yang J et al (2016) Neoadjuvant vs. adjuvant treatment of Siewert type II gastroesophageal junction cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry. J Gastrointest Oncol 7(3):403–410
Munch S, Aichmeier S, Hapfelmeier A et al (2016) Comparison of dosimetric parameters and toxicity in esophageal cancer patients undergoing 3D conformal radiotherapy or VMAT. Strahlenther Onkol 192(10):722–729
Omloo JM, Lagarde SM, Hulscher JB et al (2007) Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 246(6):992–1000 (discussion 1000–1)
Porschen R, Buck A, Fischbach W et al (2015) Z Gastroenterol 53(11):1288–1347
Ronellenfitsch U, Schwarzbach M, Hofheinz R et al (2013) Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer 49(15):3149–3158
Ronellenfitsch U, Schwarzbach M, Hofheinz R et al (2013) Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008107.pub2
Sasako M, Sano T, Yamamoto S et al (2006) Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 7(8):644–651
Schuhmacher C, Gretschel S, Lordick F et al (2010) Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 28(35):5210–5218
Shapiro J, van Lanschot JJ, Hulshof MC et al (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16(9):1090–1098
Sjoquist KM, Burmeister BH, Smithers BM et al (2011) Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 12(7):681–692
Smyth EC, Waddell TS, Cunningham D (2014) Optimal management of esophageal adenocarcinoma: should we be CROSS? J Clin Oncol 32(27):3080–3081
Stahl M, Walz MK, Stuschke M et al (2009) Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 27(6):851–856
Tepper J, Krasna MJ, Niedzwiecki D et al (2008) Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26(7):1086–1092
van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084
Ychou M, Boige V, Pignon JP et al (2011) Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29(13):1715–1721
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Münch, D. Habermehl, A. Agha, C. Belka, S.E. Combs, R. Eckel, H. Friess, A. Gerbes, N.C. Nüssler, W. Schepp, R.M. Schmid, W. Schmitt, G. Schubert-Fritschle, B. Weber, J. Werner, and J. Engel declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Stefan Münch and Daniel Habermehl contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Münch, S., Habermehl, D., Agha, A. et al. Perioperative chemotherapy vs. neoadjuvant chemoradiation in gastroesophageal junction adenocarcinoma. Strahlenther Onkol 194, 125–135 (2018). https://doi.org/10.1007/s00066-017-1225-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1225-7