Abstract
Purpose
We retrospectively evaluated the patterns of failure for robotic guided real-time breathing-motion-compensated (BMC) stereotactic body radiation therapy (SBRT) in the treatment of tumors in moving organs.
Patients and methods
Between 2011 and 2016, a total of 198 patients with 280 lung, liver, and abdominal tumors were treated with BMC-SBRT. The median gross tumor volume (GTV) was 12.3 cc (0.1–372.0 cc). Medians of mean GTV BEDα/β = 10 Gy (BED = biological effective dose) was 148.5 Gy10 (31.5–233.3 Gy10) and prescribed planning target volume (PTV) BEDα/β = 10 Gy was 89.7 Gy10 (28.8–151.2 Gy10), respectively. We analyzed overall survival (OS) and local control (LC) based on various factors, including BEDs with α/β ratios of 15 Gy (lung metastases), 21 Gy (primary lung tumors), and 27 Gy (liver metastases).
Results
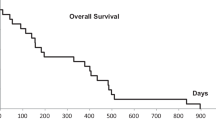
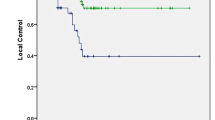
Median follow-up was 10.4 months (2.0–59.0 months). The 2‑year actuarial LC was 100 and 86.4% for primary early and advanced stage lung tumors, respectively, 100% for lung metastases, 82.2% for liver metastases, and 90% for extrapulmonary extrahepatic metastases. The 2‑year OS rate was 47.9% for all patients. In uni- and multivariate analysis, comparatively lower PTV prescription dose (equivalence of 3 × 12–13 Gy) and higher average GTV dose (equivalence of 3 × 18 Gy) to current practice were significantly associated with LC. For OS, Karnofsky performance score (100%), gender (female), and SBRT without simultaneous chemotherapy were significant prognostic factors. Grade 3 side effects were rare (0.5%).
Conclusions
Robotic guided BMC-SBRT can be considered a safe and effective treatment for solid tumors in moving organs. To reach sufficient local control rates, high average GTV doses are necessary. Further prospective studies are warranted to evaluate these points.
Zusammenfassung
Zweck
Wir führten eine retrospektive Untersuchung der Rezidivmuster bei der Behandlung von Tumoren in bewegten Organen mittels robotergeführter in Echtzeit atembewegungskompensierter (EAK) stereotaktischer Körperstammstrahlentherapie (SBRT) durch.
Patienten und Methoden
Zwischen 2011 und 2016 wurden insgesamt 198 Patienten mit 280 Lungen‑, Leber- und Abdominaltumoren mit EAK-SBRT behandelt. Das mediane makroskopische Tumorvolumen (GTV) lag bei 12,3 cm3 (0,1–372,0 cm3). Die mediane mittlere GTV-BEDα / β = 10 Gy lag bei 148,5 Gy10 (31,5–233,3 Gy10; BED = biologisch effektive Dosis) und die verschriebene PTV-BEDα / β = 10 Gy bei 89,7 Gy10 (28,8–151,2 Gy10; PTV = Planungszielvolumen). Wir analysierten das Gesamtüberleben (GÜ) und die lokale Kontrolle (LK) basierend auf verschiedenen Faktoren, einschließlich BED mit α/β-Verhältnissen von 15 Gy (Lungenmetastasen), 21 Gy (primäre Lungentumoren) und 27 Gy (Lebermetastasen).
Ergebnisse
Die mediane Nachbeobachtungszeit betrug 10,4 Monate (2,0–59,0 Monate). Die 2‑Jahres-LK betrug 100 und 86,4 % für primäre Lungentumoren im Früh- bzw. fortgeschrittenen Stadium, 100 % für Lungenmetastasen, 82,2 % für Lebermetastasen und 90 % für extrapulmonale, extrahepatische Metastasen. Die 2‑Jahres-GÜ-Rate über alle Patienten betrug 47,9 %. In der uni- und multivariaten Analyse wurde die LK vor allem durch eine zur üblichen Praxis vergleichsweise niedrige PTV-Verschreibungsdosis (äquivalent zu 3‑mal 12–13 Gy) sowie durch höhere mittlere GTV-Dosen (äquivalent zu 3‑mal 18 Gy) beeinflusst. Für ein hohes GÜ waren ein hoher Karnofsky-Index (100 %), das Geschlecht (weiblich) und die SBRT ohne gleichzeitige Chemotherapie prognostisch signifikant. Grad-3-Nebenwirkungen waren selten (0,5 %).
Schlussfolgerungen
Die robotergeführte EAK-SBRT kann als eine sichere und wirksame Behandlung für solide Tumoren in beweglichen Organen angesehen werden. Für eine ausreichend hohe lokale Kontrollrate sind hohe mittlere GTV-Dosen erforderlich. Weitere prospektive Studien sind nötig, um diese Punkte zu evaluieren.



Similar content being viewed by others
References
Guckenberger M, Allgäuer M, Appold S et al (2013) Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 8(8):1050–1058
Guckenberger M, Andratschke N, Alheit H et al (2014) Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol 190(1):26–33
Davis JN, Medbery C 3rd, Sharma S et al (2015) Stereotactic body radiotherapy for early-stage non-small cell lung cancer: clinical outcomes from a National Patient Registry. J Radiat Oncol 4(1):55–63
Guckenberger M, Klement RJ, Allgäuer M et al (2015) Local tumor control probability modeling of primary and secondary lung tumors in stereotactic body radiotherapy. Radiother Oncol. https://doi.org/10.1016/j.radonc.2015.09.008
Rieber J, Streblow J, Uhlmann L et al (2016) Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases – A pooled analysis of the German working group “stereotactic radiotherapy”. Lung Cancer 97:51–58
Tanadini-Lang S, Rieber J, Filippi AR et al (2017) Nomogram based overall survival prediction in stereotactic body radiotherapy for oligo-metastatic lung disease. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.01.003
Ricco A, Davis J, Rate W et al (2017) Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient Registry’s experience. Radiat Oncol 12(1):35–31
Sterzing F, Brunner TB, Ernst I et al (2014) Stereotactic body radiotherapy for liver tumors: principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190(10):872–881
Vautravers-Dewas C, Dewas S, Bonodeau F et al (2011) Image-guided robotic stereotactic body radiation therapy for liver metastases: is there a dose response relationship? Int J Radiat Oncol Biol Phys 81(3):e39–e47
Andratschke N, Parys A, Stadtfeld S et al (2016) Clinical results of mean GTV dose optimized robotic guided SBRT for liver metastases. Radiat Oncol 11:74
Klement RJ, Guckenberger M, Alheid H et al (2017) Stereotactic body radiotherapy for oligo-metastatic liver disease – Influence of pre-treatment chemotherapy and histology on local tumor control. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.01.013
Nuyttens JJ, Prevost JB, Van der Voort van Zijp NC et al (2007) Curative stereotactic robotic radiotherapy treatment for extracranial, extrapulmonary, extrahepatic, and extraspinal tumors: technique, early results, and toxicity. Technol Cancer Res Treat 6(6):605–610
Casamassima F, Livi L, Masciullo S et al (2012) Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys 1;82(2):919–923
Jereczek-Fossa BA, Fanetti G, Fodor C et al (2017) Salvage Stereotactic Body Radiotherapy for Isolated Lymph Node Recurrent Prostate Cancer: Single Institution Series of 94 Consecutive Patients and 124 Lymph Nodes. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2017.01.004
Liu F, Tai A, Lee P et al (2017) Tumor control probability modeling for stereotactic body radiation therapy of early-stage lung cancer using multiple bio-physical models. Radiother Oncol 122(2):286–294
Klement RJ (2017) Radiobiological parameters of liver and lung metastases derived from tumor control data of 3719 metastases. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.03.014
Kilby W, Dooley JR, Kuduvalli G et al (2010) The CyberKnife Robotic Radiosurgery System in 2010. Technol Cancer Res Treat 9(5):433–452
Schweikard A, Shiomi H, Adler J (2004) Respiration tracking in radiosurgery. Med Phys 31(10):2738–2741
Pepin EW, Wu H, Zhang Y et al (2011) Correlation and prediction uncertainties in the cyberknife synchrony respiratory tracking system. Med Phys 38(7):4036–4044
Chan MK, Werner R, Ayadi M et al (2015) Comparison of 3D and 4D Monte Carlo optimization in robotic tracking stereotactic body radiotherapy of lung cancer. Strahlenther Onkol 191(2):161–171
Chan M, Grehn M, Cremers F et al (2017) Dosimetric Implications of Residual Tracking Errors During Robotic SBRT of Liver Metastases. Int J Radiat Oncol Biol Phys 97(4):839–848
Lacornerie T, Lisbona A, Mirabel X et al (2014) GTV-based prescription in SBRT for lung lesions using advanced dose calculation algorithms. Radiat Oncol 9(16):223
Schlaefer A, Schweikard A (2008) Stepwise multi-criteria optimization for robotic radiosurgery. Med Phys 35(5):2094–2103
Blanck O, Wang L, Baus W et al (2016) Inverse Treatment Planning for Spinal Robotic Radiosurgery: An International Multi-Institutional Benchmark Trial. J Appl Clin Med Phys 17(3):313–330
Deng J, Guerrero T, Ma CM et al (2004) Modelling 6 MV photon beams of a stereotactic radiosurgery system for Monte Carlo treatment planning. Phys Med Biol 7;49(9):1689–1704 (May)
Grimm J, LaCouture T, Croce R et al (2011) Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 12(2):3368–3368 (Review)
Trakul N, Chang CN, Harris J et al (2012) Tumor volume-adapted dosing in stereotactic ablative radiotherapy of lung tumors. Int J Radiat Oncol Biol Phys 1;84(1):231–237 (Sep)
Ernst F, Dürichen R, Schlaefer A, Schweikard A (2013) Evaluating and comparing algorithms for respiratory motion prediction. Phys Med Biol 58(11):3911–3929
Malinowski KT, McAvoy TJ, George R et al (2012) Mitigating errors in external respiratory surrogate-based models of tumor position. Int J Radiat Oncol Biol Phys 82(5):e709–e716
Bibault JE, Prevost B, Dansin E et al (2012) Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat Oncol 7(24):102
Malinowski K, McAvoy TJ, George R et al (2013) Maintaining tumor targeting accuracy in real-time motion compensation systems for respiration-induced tumor motion. Med Phys 40(7):71709
Mantel F, Flentje M, Guckenberger M (2013) Stereotactic body radiation therapy in the re-irradiation situation – a review. Radiat Oncol 8(5):7
Ceylan C, Hamacı A, Ayata H et al (2017) Re-Irradiation of Locoregional NSCLC Recurrence Using Robotic Stereotactic Body Radiotherapy. Oncol Res Treat 40(4):207–214
Trovo M, Minatel E, Durofil E et al (2014) Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys 88(5):1114–1119
Giuliani ME, Hope A, Mangona V et al (2017) Predictors and Patterns of Regional Recurrence Following Lung SBRT: A Report From the Elekta Lung Research Group. Clin Lung Cancer 18(2):162–168
Nieder C, De Ruysscher D, Gaspar LE et al (2017) Reirradiation of recurrent node-positive non-small cell lung cancer after previous stereotactic radiotherapy for stage I disease : A multi-institutional treatment recommendation. Strahlenther Onkol. https://doi.org/10.1007/s00066-017-1130-0
Bibault JE, Mirabel X, Lacornerie T et al (2015) Adapted Prescription Dose for Monte Carlo Algorithm in Lung SBRT: Clinical Outcome on 205 Patients. PLOS ONE 10(7):e133617
van der Voort van Zyp NC, Hoogeman MS, van de Water S et al (2010) Clinical introduction of Monte Carlo treatment planning: a different prescription dose for non-small cell lung cancer according to tumor location and size. Radiother Oncol 96(1):55–60
Iwata H, Ishikura S, Murai T et al (2017) A phase I/II study on stereotactic body radiotherapy with real-time tumor tracking using CyberKnife based on the Monte Carlo algorithm for lung tumors. Int J Clin Oncol. https://doi.org/10.1007/s10147-017-1123-0
Zheng D, Zhu X, Zhang Q et al (2016) Target dose conversion modeling from pencil beam (PB) to Monte Carlo (MC) for lung SBRT. Radiat Oncol 11:83
Dewas S, Bibault JE, Mirabel X et al (2012) Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy. Radiat Oncol 7:166
Chang DT, Swaminath A, Kozak M et al (2011) Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 117(17):4060–4069
Wahl DR, Stenmark MH, Tao Y et al (2016) Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 34(5):452–459
Peterson J, Niles C, Patel A et al (2016) Stereotactic Body Radiotherapy for Large (〉 5 cm) Non-Small-Cell Lung Cancer. Clin Lung Cancer. https://doi.org/10.1016/j.cllc.2016.11.020
Tanadini-Lang S, Rieber J, Filippi AR et al (2017) Nomogram based overall survival prediction in stereotactic body radiotherapy for oligo-metastatic lung disease. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.01.003
Rieber J, Abbassi-Senger N, Adebahr S et al (2016) Influence of Institutional Experience and Technological Advances on Outcome of Stereotactic Body Radiation Therapy for Oligometastatic Lung Disease. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2016.09.026
Klement RJ, Allgäuer M, Appold S et al (2014) Support vector machine-based prediction of local tumor control after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 88(3):732–738
Klement RJ, Allgäuer M, Andratschke N et al (2016) Bayesian Cure Rate Modeling of Local Tumor Control: Evaluation in Stereotactic Body Radiation Therapy for Pulmonary Metastases. Int J Radiat Oncol Biol Phys 94(4):841–849
Acknowledgements
The authors would kindly thank Rainer Klement (Schweinfurt, Germany) for his helpful comments and discussions on biological effective dose calculation. The authors would also like to thank Prof. Dr. Jost Philipp Schäfer (Kiel, Germany), PD Dr. Peter Hunold (Lübeck, Germany), Dr. Gunnar Gaffke (Güstrow, Germany), Dr. Klaus-Rainer Bogun (Rostock, Germany), Prof. Dr. Norbert Hosten (Greifswald), PD Dr. Nikolaos Tselis (Frankfurt, Germany) and Prof. Thomas Vogl (Frankfurt, Germany) for implanting the fiducials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Stera, P. Balermpas, M.K.H. Chan, S. Huttenlocher, S. Wurster, C. Keller, D. Imhoff, D. Rades, J. Dunst, C. Rödel, G. Hildebrandt and O. Blanck declare that they have no competing interests.
Ethical standards
This retrospective analysis was approved by the local ethics committee of the medical faculty of the university Frankfurt (477/15), Rostock (A2016-0008) and Lübeck (13–218 A).
Rights and permissions
About this article
Cite this article
Stera, S., Balermpas, P., Chan, M.K.H. et al. Breathing-motion-compensated robotic guided stereotactic body radiation therapy. Strahlenther Onkol 194, 143–155 (2018). https://doi.org/10.1007/s00066-017-1204-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1204-z