Abstract
Purpose
The purpose of this study was to evaluate postoperative radiotherapy regarding outcome and toxicity in patients with thymic epithelial tumors (TET) after surgery.
Materials and methods
We retrospectively analyzed medical records of 41 patients with TET treated with postoperative radiotherapy at our institution between 1995 and 2012. The impact of prognostic factors (e.g., Masaoka stage, histological subtype) was investigated and radiation-related toxicity was assessed.
Results
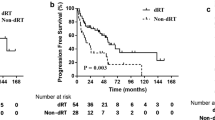
Median age was 59.8 years and median follow-up was 61 months. In 24.4 %, TETs were associated with paraneoplastic syndromes. The 5-year overall survival (OS) was 89.5 % and the 5-year disease-free survival (DFS) was 88.9 %. Masaoka stage had a significant impact on OS (p = 0.007). Locally limited stages I + II had a 5-year OS of 100 % compared to 80 % for stage III and 66.7 % for stage IV. The 5-year DFS was excellent with 100 % for both WHO groups A/AB/B1 and B2, respectively, and significantly (p = 0.005) differed from B3/C-staged patients with a 5-year DFS of 63.6 %. Resection status, paraneoplastic association, radiation dose, or tumor size did not influence survival. There were no high-grade acute or late side effects caused by radiotherapy.
Conclusion
Masaoka stage has a significant impact on OS as WHO type has on DFS in patients with TETs after surgery and adjuvant irradiation. Postoperative radiotherapy with doses around 50 Gy is safe and not likely to cause high-grade toxicity. Further prospective trials are necessary to separate patient subgroups that benefit from radiotherapy from those that do not.
Zusammenfassung
Ziel
Die vorliegende Studie hatte zum Ziel, die postoperative Radiotherapie von Patienten nach Resektion einer Thymusneoplasie epithelialen Ursprungs (Thymom, Thymuskarzinom, „thymic epithelial tumors“, TET) hinsichtlich prognostischer Relevanz und Nebenwirkungsprofil zu beurteilen.
Material und methoden
Wir analysierten retrospektiv die medizinischen Krankenakten von 41 Patienten mit TET, die zwischen 1995 und 2012 eine postoperative Radiotherapie in unserer Einrichtung erhielten. Hierbei untersuchten wir insbesondere mögliche Prognosefaktoren wie Masaoka-Stadium, histologischen Subtyp sowie weitere und erfassten radiogene Nebenwirkungen.
Ergebnisse
Das mediane Alter betrug 59,8 Jahre, das mediane Follow-up lag bei 61 Monaten. Bei 24,4 % aller Patienten trat ein paraneoplastisches Syndrom auf. Das 5-Jahres-Gesamtüberleben lag bei 89,5 %, das krankheitsfreie 5-Jahres-Übeleben bei 88,9 %. Das Masaoka-Stadium hatte signifikanten Einfluss auf das Gesamtüberleben (p = 0,007). Die lokal begrenzten Stadien I + II hatten ein 5-Jahres-Gesamtüberleben von 100 % im Vergleich zu 80 % bei Masaoka III und 66,7 % bei Masaoka IV. Das krankheitsfreie 5-Jahres-Übeleben war mit jeweils 100 % in den WHO-Gruppen A/AB/B1 bzw. B2 exzellent und damit signifikant besser (p = 0,005) im Vergleich zu 63,6 % bei Patienten mit B3/C. Resektionsstatus, Paraneoplasien, Bestrahlungsdosis oder Tumorgröße hatten keinen Einfluss auf das Überleben. Es traten keine höhergradigen, radiogenen Akut- oder Spätnebenwirkungen auf.
Schlussfolgerung
Das Masaoka-Stadium hat signifikanten Einfluss auf das Gesamtüberleben und der WHO-Subtyp auf das krankheitsfreie Überleben bei Patienten mit postoperativer Radiotherapie nach Resektion eines TET. Eine Bestrahlungsdosis von ca. 50 Gy kann hierbei sicher und ohne besonderes Risiko für höhergradige Nebenwirkungen appliziert werden. Allerdings werden weitere, prospektive Studien benötigt, um zu differenzieren, welche Patientensubgruppen von einer adjuvanten Radiotherapie profitieren und welche nicht.




Similar content being viewed by others
References
Engels EA, Pfeiffer RM (2003) Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 105:546–551
Chen YD, Feng QF, Lu HZ et al (2010) Role of adjuvant radiotherapy for stage II thymoma after complete tumor resection. Int J Radiat Oncol Biol Phys 78:1400–1406
Zhu G, He S, Fu X et al (2004) Radiotherapy and prognostic factors for thymoma: a retrospective study of 175 patients. Int J Radiat Oncol Biol Phys 60:1113–1119
Kondo K, Monden Y (2003) Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 76:878–884 (discussion 884–875)
Curran WJ Jr, Kornstein MJ, Brooks JJ et al (1988) Invasive thymoma: the role of mediastinal irradiation following complete or incomplete surgical resection. J Clin Oncol 6:1722–1727
Forquer JA, Rong N, Fakiris AJ et al (2010) Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys 76:440–445
Fuller CD, Ramahi EH, Aherne N et al (2010) Radiotherapy for thymic neoplasms. J Thorac Oncol 5:S 327–335
Korst RJ, Kansler AL, Christos PJ et al (2009) Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 87:1641–1647
Masaoka A, Monden Y, Nakahara K et al (1981) Follow-up study of thymomas with special reference to their clinical stages. Cancer 48:2485–2492
Masaoka A, Yamakawa Y, Niwa H et al (1994) Thymectomy and malignancy. Eur J Cardiothorac Surg 8:251–253
Rosai J (1999) Histological typing of tumours of the thymus. Springer, New York
Strobel P, Marx A, Zettl A et al (2005) Thymoma and thymic carcinoma: an update of the WHO Classification 2004. Surg Today 35:805–811
Levine GD, Rosai J (1978) Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol 9:495–515
Marchevsky AM, Gupta R, Mckenna RJ et al (2008) Evidence-based pathology and the pathologic evaluation of thymomas: the World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer 112:2780–2788
Wu KL, Mao JF, Chen GY et al (2009) Prognostic predictors and long-term outcome of postoperative irradiation in thymoma: a study of 241 patients. Cancer Invest 27:1008–1015
Patel S, Macdonald OK, Nagda S et al (2012) Evaluation of the role of radiation therapy in the management of malignant thymoma. Int J Radiat Oncol Biol Phys 82:1797–1801
Kundel Y, Yellin A, Popovtzer A et al (2007) Adjuvant radiotherapy for thymic epithelial tumor: treatment results and prognostic factors. Am J Clin Oncol 30:389–394
Chang JH, Kim HJ, Wu HG et al (2011) Postoperative radiotherapy for completely resected stage II or III thymoma. J Thorac Oncol 6:1282–1286
Harnath T, Marx A, Strobel P et al (2012) Thymoma—a clinico-pathological long-term study with emphasis on histology and adjuvant radiotherapy dose. J Thorac Oncol 7:1867–1871
Ogawa K, Uno T, Toita T et al (2002) Postoperative radiotherapy for patients with completely resected thymoma: a multi-institutional, retrospective review of 103 patients. Cancer 94:1405–1413
Strobel P, Bauer A, Puppe B et al (2004) Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 22:1501–1509
Vassiliou V, Tsamandas A, Katodritis N et al (2009) The role of postoperative radiotherapy in the management of patients with thymic tumors—a retrospective study. In Vivo 23:843–852
Singhal S, Shrager JB, Rosenthal DI et al (2003) Comparison of stages I–II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 76:1635–1641 (discussion 1641–1632)
Berman AT, Litzky L, Livolsi V et al (2011) Adjuvant radiotherapy for completely resected stage 2 thymoma. Cancer 117:3502–3508
Mangi AA, Wright CD, Allan JS et al (2002) Adjuvant radiation therapy for stage II thymoma. Ann Thorac Surg 74:1033–1037
Fan C, Feng Q, Chen Y et al (2013) Postoperative radiotherapy for completely resected Masaoka stage III thymoma: a retrospective study of 65 cases from a single institution. Radiat Oncol 8:199
Weksler B, Shende M, Nason KS et al (2012) The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg 93:1822–1828 (discussion 1828–1829)
Mangi AA, Wain JC, Donahue DM et al (2005) Adjuvant radiation of stage III thymoma: is it necessary? Ann Thorac Surg 79:1834–1839
Myojin M, Choi NC, Wright CD et al (2000) Stage III thymoma: pattern of failure after surgery and postoperative radiotherapy and its implication for future study. Int J Radiat Oncol Biol Phys 46:927–933
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Calvo FA, Sole CV, Serrano J et al (2014) Preoperative chemoradiation with or without induction oxaliplatin plus 5-fluorouracil in locally advanced rectal cancer: long-term outcome analysis. Strahlenther Onkol 190:149–157
Kunitoh H, Tamura T, Shibata T et al (2010) A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 103:6–11
Korst RJ, Bezjak A, Blackmon S et al (2014) Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 147:36–44, 46 (e1)
Hsu HC, Huang EY, Wang CJ et al (2002) Postoperative radiotherapy in thymic carcinoma: treatment results and prognostic factors. Int J Radiat Oncol Biol Phys 52:801–805
Eng TY, Fuller CD, Jagirdar J et al (2004) Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 59:654–664
Tormoehlen LM, Pascuzzi RM (2008) Thymoma, myasthenia gravis, and other paraneoplastic syndromes. Hematol Oncol Clin North Am 22:509–526
Fakhrian K, Oechsner M, Kampfer S et al (2013) Advanced techniques in neoadjuvant radiotherapy allow dose escalation without increased dose to the organs at risk: Planning study in esophageal carcinoma. Strahlenther Onkol 189:293–300
Song C, Pyo H, Kim J et al (2012) Superiority of conventional intensity-modulated radiotherapy over helical tomotherapy in locally advanced non-small cell lung cancer. A comparative plan analysis. Strahlenther Onkol 188:901–909
Butof R, Kirchner K, Appold S et al (2014) Potential clinical predictors of outcome after postoperative radiotherapy of non-small cell lung cancer. Strahlenther Onkol 190:263–269
Latz D, Schraube P, Oppitz U et al (1997) Invasive thymoma: treatment with postoperative radiation therapy. Radiology 204:859–864
Compliance with ethical guidelines
Conflict of interest
M.F. Häfner, F. Roeder, F. Sterzing, D. Krug, S.A. Koerber, J. Kappes, H. Hoffmann, A. Slynko, J. Debus, and M. Bischof state that there are no conflicts of interest. All studies on patient data were carried out with approval of the institutional ethical review committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Häfner, M., Roeder, F., Sterzing, F. et al. Postoperative radiotherapy of patients with thymic epithelial tumors (TET). Strahlenther Onkol 191, 133–140 (2015). https://doi.org/10.1007/s00066-014-0740-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0740-z