Abstract
Purpose
Epigenetic profiling has recently identified clinically and molecularly distinct subgroups of ependymoma. The 2016 World Health Organization (WHO) classification recognized supratentorial ependymomas (ST-EPN) with REL-associated protein/p65 (RELA) fusion as a clinicopathological entity. These tumors represent 70% of pediatric ST-EPN characterized by recurrent C11orf95-RELA fusion transcripts, which lead to pathological activation of the nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells (NF-κB) signaling pathway. Cyclin-dependent kinase inhibitor 2A (CDKN2A) inactivation has also been reported to correlate with poor prognosis. Here, we systematically describe magnetic resonance imaging (MRI) characteristics of RELA-fused ST-EPN, with respect to CDKN2A deletion status.
Methods
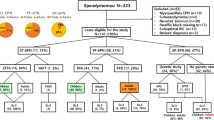
Our cohort of patients with ST-EPN (n = 57) was obtained from the database of the German Brain Tumor Reference Center of the German Society for Neuropathology and Neuroanatomy (DGNN), and tumors were diagnosed according to the 2016 WHO classification. Molecular characterization identified 47 RELA-fused tumors. We analyzed the preoperative MRI according to standardized criteria, and comparison was performed between CDKN2A altered (n = 21) and CDKN2A wild type (n = 26) tumors.
Results
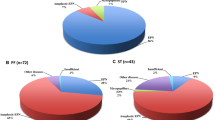
The RELA-fused ST-EPN showed a spectrum of predominantly hemispheric tumors with cysts and necrosis. Statistical analysis on CDKN2A status revealed significant differences in terms of younger manifestation age (p =0.002) and more intratumoral hemorrhage in T2-weighted imaging (T2WI) (p =0.010) in wild type tumors; however, the location was not a parameter for differentiation.
Conclusion
This study first provides comprehensive MRI data for RELA-fused ST-EPN as a distinct entity, with further interest on CDKN2A genomic status. Patient stratification by morphological MRI alone seems difficult at present. The results may support ongoing research in ST-EPN within the framework of the radiogenomics concept.




Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–35.
Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, Wani K, Tatevossian R, Punchihewa C, Johann P, Reimand J, Warnatz HJ, Ryzhova M, Mack S, Ramaswamy V, Capper D, Schweizer L, Sieber L, Wittmann A, Huang Z, van Sluis P, Volckmann R, Koster J, Versteeg R, Fults D, Toledano H, Avigad S, Hoffman LM, Donson AM, Foreman N, Hewer E, Zitterbart K, Gilbert M, Armstrong TS, Gupta N, Allen JC, Karajannis MA, Zagzag D, Hasselblatt M, Kulozik AE, Witt O Collins VP, von Hoff K, Rutkowski S, Pietsch T, Bader G, Yaspo ML, von Deimling A, Lichter P, Taylor MD, Gilbertson R, Ellison DW, Aldape K, Korshunov A, Kool M, Pfister SM. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27:728–43.
Pajtler KW, Pfister SM, Kool M. Molecular dissection of ependymomas. Oncoscience. 2015;2:827–8.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Korshunov A, Golanov A, Timirgaz V. p14ARF protein (FL-132) immunoreactivity in intracranial ependymomas and its prognostic significance: an analysis of 103 cases. Acta Neuropathol. 2001;102:271–7.
Korshunov A, Witt H, Hielscher T, Benner A, Remke M, Ryzhova M, Milde T, Bender S, Wittmann A, Schöttler A, Kulozik AE, Witt O, von Deimling A, Lichter P, Pfister S. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol. 2010;28:3182–90.
Milde T, Kleber S, Korshunov A, Witt H, Hielscher T, Koch P, Kopp HG, Jugold M, Deubzer HE, Oehme I, Lodrini M, Gröne HJ, Benner A, Brüstle O, Gilbertson RJ, von Deimling A, Kulozik AE, Pfister SM, Martin-Villalba A, Witt O. A novel human high-risk ependymoma stem cell model reveals the differentiation-inducing potential of the histone deacetylase inhibitor Vorinostat. Acta Neuropathol. 2011;122:637–50.
Milde T, Pfister S, Korshunov A, Deubzer HE, Oehme I, Ernst A, Starzinski-Powitz A, Seitz A, Lichter P, von Deimling A, Witt O. Stepwise accumulation of distinct genomic aberrations in a patient with progressively metastasizing ependymoma. Genes Chromosomes Cancer. 2009;48:229–38.
Ellingson BM. Radiogenomics and imaging phenotypes in glioblastoma: novel observations and correlation with molecular characteristics. Curr Neurol Neurosci Rep. 2015;15:506.
Gevaert O, Mitchell LA, Achrol AS, Xu J, Echegaray S, Steinberg GK, Cheshier SH, Napel S, Zaharchuk G, Plevritis SK. Glioblastoma multiforme: exploratory radiogenomic analysis by using quantitative image features. Radiology. 2014;273:168–74.
Perreault S, Ramaswamy V, Achrol AS, Chao K, Liu TT, Shih D, Remke M, Schubert S, Bouffet E, Fisher PG, Partap S, Vogel H, Taylor MD, Cho YJ, Yeom KW. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35:1263–9.
Pajtler KW, Mack SC, Ramaswamy V, Smith CA, Witt H, Smith A, Hansford JR, von Hoff K, Wright KD, Hwang E, Frappaz D, Kanemura Y, Massimino M, Faure-Conter C, Modena P, Tabori U, Warren KE, Holland EC, Ichimura K, Giangaspero F, Castel D, von Deimling A, Kool M, Dirks PB, Grundy RG, Foreman NK, Gajjar A, Korshunov A, Finlay J, Gilbertson RJ, Ellison DW, Aldape KD, Merchant TE, Bouffet E, Pfister SM3, Taylor MD. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133:5–12.
Figarella-Branger D, Lechapt-Zalcman E, Tabouret E, Jünger S, de Paula AM, Bouvier C, Colin C, Jouvet A, Forest F, Andreiuolo F, Quintin-Roue I, Machet MC, Heitzmann A, Milin S, Sevestre H, Godfraind C, Labrousse F, Metellus P, Scavarda D, Pietsch T. Supratentorial clear cell ependymomas with branching capillaries demonstrate characteristic clinicopathological features and pathological activation of nuclear factor-kappaB signaling. Neuro Oncol. 2016;18:919-27.
Pietsch T, Wohlers I, Goschzik T, Dreschmann V, Denkhaus D, Dörner E, Rahmann S, Klein-Hitpass L. Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-kappaB signaling pathway. Acta Neuropathol. 2014;127:609–11.
Japp AS, Gessi M, Messing-Jünger M, Denkhaus D, Zur Mühlen A, Wolff JE, Hartung S, Kordes U, Klein-Hitpass L, Pietsch T. High-resolution genomic analysis does not qualify atypical plexus papilloma as a separate entity among choroid plexus tumors. J Neuropathol Exp Neurol. 2015;74:110–20.
Wang Y, Cottman M, Schiffman JD. Molecular inversion probes: a novel microarray technology and its application in cancer research. Cancer Genet. 2012;205:341–55.
Aboian MS, Solomon DA, Felton E, Mabray MC, Villanueva-Meyer JE, Mueller S, Cha S. Imaging characteristics of pediatric diffuse midline gliomas with histone H3 K27M mutation. AJNR Am J Neuroradiol. 2017;38:795-800.
Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y, Lee R, Tatevossian RG, Phoenix TN, Thiruvenkatam R, White E, Tang B, Orisme W, Gupta K, Rusch M, Chen X, Li Y, Nagahawhatte P, Hedlund E, Finkelstein D, Wu G, Shurtleff S, Easton J, Boggs K, Yergeau D, Vadodaria B, Mulder HL, Becksfort J, Gupta P, Huether R, Ma J, Song G, Gajjar A, Merchant T, Boop F, Smith AA, Ding L, Lu C, Ochoa K, Zhao D, Fulton RS, Fulton LL, Mardis ER, Wilson RK, Downing JR, Green DR, Zhang J, Ellison DW, Gilbertson RJ. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506(7489):451–5.
Kaatsch P, Spix C. German childhood cancer registry—Annual Report 2015 (1980–2014). Mainz: Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz; 2015.
Poussaint TY. Magnetic resonance imaging of pediatric brain tumors: state of the art. Top Magn Reson Imaging. 2001;12:411–33.
Osborn AG. Osborn’s brain: imaging, pathology, and anatomy. Salt Lake City: Amirsys; 2013. pp. 501–4.
Bühren J, Christoph AH, Buslei R, Albrecht S, Wiestler OD, Pietsch T. Expression of the neurotrophin receptor p75NTR in medulloblastomas is correlated with distinct histological and clinical features: evidence for a medulloblastoma subtype derived from the external granule cell layer. J Neuropathol Exp Neurol. 2000;59:229–40.
Massimino M, Biassoni V, Gandola L, Garrè ML, Gatta G, Giangaspero F, Poggi G, Rutkowski S. Childhood medulloblastoma. Crit Rev Oncol Hematol. 2016;105:35–51.
Nowak J, Seidel C, Pietsch T, Alkonyi B, Fuss TL, Friedrich C, von Hoff K, Rutkowski S, Warmuth-Metz M. Systematic comparison of MRI findings in pediatric ependymoblastoma with ependymoma and CNS primitive neuroectodermal tumor not otherwise specified. Neuro Oncol. 2015;17:1157-65.
Osborn AG, Daines JH, Wing SD. The evaluation of ependymal and subependymal lesions by cranial computed tomography. Radiology. 1978;127:397–401.
Acknowledgements
This work was supported by the German Childhood Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Nowak, S.T. Jünger, H. Huflage, C. Seidel, A. Hohm, L.A. Vandergrift, K. von Hoff, S. Rutkowski, T. Pietsch and M. Warmuth-Metz declare that they have no competing interests.
Caption Electronic Supplementary Material
62_2018_704_MOESM1_ESM.jpg
Fig. S1: Representative histology and immunohistochemical reaction for p65RelA and p16 protein expression in two cases with RELA-positive anaplastic ependymomas (WHO grade III). HE (Hematoxylin/Eosin) histology shows vascular proliferations in both cases (arrows). Both samples display the characteristic nuclear accumulation of p65RelA. Case #1 lost p16 protein indicating CDKN2A gene loss. p16 is only detected in normal cells such as endothelial cells (arrow). In contrast, case #2 shows retained p16 expression in the tumor cell nuclei (arrow)
62_2018_704_MOESM2_ESM.jpg
Fig. S2: MR images of two RELA-fused ST-EPN with and without CDKN2A deletion. Patient 1 (A–C, CDKN2A deleted = del, GE Signa 1.5 T), manifestation age: 9yr 7 m, with no signs for hemorrhage. Patient 2 (D–F, CDKN2A wild type = WT, Siemens Vision 1.5 T), manifestation age: 5yr 5 m, shows signs for hemorrhage in T2WI (white arrow in E), confirmed in T1 (D) and T2* (F)
Rights and permissions
About this article
Cite this article
Nowak, J., Jünger, S.T., Huflage, H. et al. MRI Phenotype of RELA-fused Pediatric Supratentorial Ependymoma. Clin Neuroradiol 29, 595–604 (2019). https://doi.org/10.1007/s00062-018-0704-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-018-0704-2