Abstract
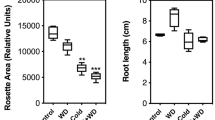
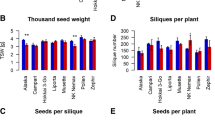
Plants can alter physiological and developmental trajectories in response to environmental cues by means of phenotypic plasticity. While cases of immediate plastic responses to different environments are well studied, phenotypic changes can also be delayed and occur in later life cycle stages. In this study, we investigated latent phenotypic plasticity in the development and chemical profile of Brassica rapa plants exposed to transient stress as seedlings. Four different stresses were applied to germinating seedlings: salinity, drought, nutrient deficiency, or acidity. Growth, reproduction, and glucosinolate chemical defenses (in leaves and seeds) were measured over the plants’ life cycles after normal conditions were restored. Despite initial stunting, B. rapa individuals recovered in total stem length and seed count compared with unstressed controls. There were, however, latent responses in flowering time, which was delayed in salinity-, drought-, or acid-stressed plants. Reductions in total flower count and total seed pod count were also observed in nutrient-deficiency stressed plants. Strikingly, previously stressed plants also showed latent differences in glucosinolate chemical defenses. Acid-stressed plants had higher concentrations of the plants’ major glucosinolate, gluconapin (3-butenylglucosinolate), in leaves and seeds, while nutrient deficiency-stressed plants had lower seed levels of gluconapin. Our experiments show that, despite outward recovery of growth, previously stressed B. rapa plants alter later defense and reproduction, leading to a plastic response delayed across life cycle stages. Thus, even transient, recoverable stress can have latent consequences for ecologically important chemical traits.




Similar content being viewed by others
References
Agerbirk N, Petersen BL, Olsen CE, Halkier BA, Nielsen JK (2001) 1,4-Dimethoxyglucobrassicin in Barbarea and 4-hydroxyglucobrassicin in Arabidopsis and Brassica. J Agric Food Chem 49(3):1502–1507
Agerbirk N, Olsen CE, Topbjerg HB, Sørensen JC (2007) Host plant-dependent metabolism of 4-hydroxybenzylglucosinolate in Pieris rapae: Substrate specificity and effects of genetic modification and plant nitrile hydratase. Insect Biochem Mol Biol 37(11):1119–1130
Agerbirk N, Warwick SI, Hansen PR, Olsen CE (2008) Sinapis phylogeny and evolution of glucosinolates and specific nitrile degrading enzymes. Phytochemistry 69(17):2937–2949. doi:10.1016/j.phytochem.2008.08.014
Agerbirk N, De Vos M, Kim J, Jander G (2009) Indole glucosinolate breakdown and its biological effects. Phytochem Rev 8(1):101–120. doi:10.1007/s11101-008-9098-0
Agerbirk N, Chew F, Olsen C, Jørgensen K (2010) Leaf and floral parts feeding by orange tip butterfly larvae depends on larval position but not on glucosinolate profile or nitrogen level. J Chem Ecol 36(12):1335–1345. doi:10.1007/s10886-010-9880-5
Agrawal AA (2000) Specificity of induced resistance in wild radish: causes and consequences for two specialist and two generalist caterpillars. Oikos 89(3):493–500
Agrawal AA (2001) Transgenerational consequences of plant responses to herbivory: an adaptive maternal effect? Am Nat 157(5):555–569
Badenes-Perez FR, Reichelt M, Heckel DG (2010) Can sulfur fertilisation improve the effectiveness of trap crops for diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae)? Pest Manage Sci 66(8):832–838. doi:10.1002/ps.1949
Badenes-Pérez FR, Reichelt M, Gershenzon J, Heckel DG (2010) Phylloplane location of glucosinolates in Barbarea spp. (Brassicaceae) and misleading assessment of host suitability by a specialist herbivore. New Phytol 189(2):549–556
Bednarek P, Osbourn A (2009) Plant-microbe interactions: chemical diversity in plant defense. Science 324(5928):746–748
Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323(5910):101–106. doi:10.1126/science.1163732
Boege K, Marquis RJ (2005) Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol Evol 20(8):441–448
Bonser AM, Lynch J, Snapp S (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol 132(2):281–288
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173(6):603–608. doi:10.1016/j.plantsci.2007.09.002
Champolivier L, Merrien A (1996) Effects of water stress applied at different growth stages to Brassica napus L. var. oleifera on yield, yield components and seed quality. Eur J Agron 5(3–4):153–160
Chinnusamy V, Zhu JK (2009) Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12(2):133–139
Choi CS, Sano H (2007) Abiotic-stress induces demethylation and transcriptional activation of a gene encoding a glycerophosphodiesterase-like protein in tobacco plants. Mol Genet Genomics 277(5):589–600
De Block M, Stoks R (2008) Short-term larval food stress and associated compensatory growth reduce adult immune function in a damselfly. Ecol Entomol 33(6):796–801
Donaldson JR, Lindroth RL (2008) Effects of variable phytochemistry and budbreak phenology on defoliation of aspen during a forest tent caterpillar outbreak. Agric For Entomol 10(4):399–410. doi:10.1111/j.1461-9563.2008.00392.x
Droste T, Flory SL, Clay K (2010) Variation for phenotypic plasticity among populations of an invasive exotic grass. Plant Ecol 207:297–306
Durrant WE, Dong X (2004) Systemic acquired resistance. Annual review of Phytopathology, vol 42
Falk KL, Tokuhisa JG, Gershenzon J (2007) The effect of sulfur nutrition on plant glucosinolate content: physiology and molecular mechanisms. Plant Biol 9(5):573–581
Fan J, Crooks C, Creissen G, Hill L, Fairhurst S, Doerner P, Lamb C (2011) Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science 331(6021):1185–1188. doi:10.1126/science.1199707
Frost CJ, Appel HM, Carlson JE, De Moraes CM, Mescher MC, Schultz JC (2007) Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett 10(6):490–498
Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146(3):818–824
Giamoustaris A, Mithen R (1997) Glucosinolates and disease resistance in oilseed rape (Brassica napus ssp. oleifera). Plant Pathol 46(2):271–275
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333. doi:10.1146/annurev.arplant.57.032905.105228
Hashida SN, Uchiyama T, Martin C, Kishima Y, Sano Y, Mikami T (2006) The temperature-dependent change in methylation of the Antiirhinum transposon Tam3 is controlled by the activity of its transposase. Plant Cell 18(1):104–118
Heil M, Bostock RM (2002) Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann Bot 89(5):503–512
Hopkins RJ, Van Dam NM, Van Loon JJA (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. vol 54
Jenks MA, Hasegawa PM (2005) Plant abiotic stress. Biological sciences series. Blackwell, Oxford
Karban R, Baldwin IT (1997) Induced responses to herbivory. Interspecific interactions. University of Chicago Press, Chicago
Kerley SJ (2000) Changes in root morphology of white lupin (Lupinus albus L.) and its adaptation to soils with heterogeneous alkaline/acid profiles. Plant Soil 218(1–2):197–205
Khan T, Khan K, Haq MZU (2005) Effect of storage fungi on the seed quality parameters of different mustard varieties. Pak J Sci Ind Res 48(5):362–365
Kim SJ, Matsuo T, Watanabe M, Watanabe Y (2002) Effect of nitrogen and sulphur application on the glucosinolate content in vegetable turnip rape (Brassica rapa L.). Soil Sci Plant Nutr 48(1):43–49
Labra M, Ghiani A, Citterio S, Sgorbati S, Sala F, Vannini C, Ruffini-Castiglione M, Bracale M (2002) Analysis of cytosine methylation pattern in response to water deficit in pea root tips. Plant Biol 4(6):694–699
Lammerink J, MacGibbon DB, Wallace AR (1984) Effect of the cabbage aphid (Brevicoryne brassicae) on total glucosinolate in the seed of oilseed rape (Brassica napus). N Z J Agric Res 27:89–92
Lopez-Berenguer C, Martinez-Ballesta MC, Garcia-Viguera C, Carvajal M (2008) Leaf water balance mediated by aquaporins under salt stress and associated glucosinolate synthesis in broccoli. Plant Sci 174(3):321–328
Musgrave ME (2000) Realizing the potential of rapid-cycling Brassica as a model system for use in plant biology research. J Plant Growth Regul 19(3):314–325
Nilsen ET, Orcutt DM (1996) The physiology of plants under stress—abiotic factors. Wiley, New York
Padilla G, Cartea ME, Velasco P, de Haro A, Ordás A (2007) Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 68(4):536–545
Pahkala M, Laurila A, Merilä J (2001) Carry-over effects of ultraviolet-B radiation on larval fitness in Rana temporaria. Proc Royal Soc B: Biol Sci 268(1477):1699–1706
Pechenik JA (2006) Larval experience and latent effects—metamorphosis is not a new beginning. Integr Comp Biol 46(3):323–333. doi:10.1093/Icb/Icj028
Pechenik JA, Rittschof D, Schmidt AR (1993) Influence of delayed metamorphosis on survival and growth of juvenile barnacles Balanus amphitrite. Mar Biol 115(2):287–294
Pechenik JA, Jarrett JN, Rooney J (2002) Relationships between larval nutritional experience, larval growth rates, juvenile growth rates, and juvenile feeding rates in the prosobranch gastropod Crepidula fornicata. J Exp Mar Biol Ecol 280(1–2):63–78
Pigliucci M (1997) Ontogenetic phenotypic plasticity during the reproductive phase in Arabidopsis thaliana (brassicaceae). Am J Bot 84(7):887–895
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH (eds) Herbivores, their interaction with secondary plant metabolites. Academic Press, Boston, pp 1–55
Sack L, Grubb PJ (2002) The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia 131(2):175–185
Schlichting CD (2002) Phenotypic plasticity in plants. Plant Species Biol 17(2–3):85–88
Smallegange R, van Loon J, Blatt S, Harvey J, Agerbirk N, Dicke M (2007) Flower vs. leaf feeding by Pieris brassicae: glucosinolate-rich flower tissues are preferred and sustain higher growth rate. J Chem Eco 33(10):1831–1844. doi:10.1007/s10886-007-9350-x
Soler R, Bezemer TM, Van Der Putten WH, Vet LEM, Harvey JA (2005) Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J Anim Ecol 74(6):1121–1130
Sønderby IE, Burow M, Rowe HC, Kliebenstein DJ, Halkier BA (2010) A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol 153(1):348–363. doi:10.1104/pp.109.149286
St. Clair SB, Monson SD, Smith EA, Cahill DG, Calder WJ (2009) Altered leaf morphology, leaf resource dilution and defense chemistry induction in frost-defoliated aspen (Populus tremuloides). Tree Physiol 29(10):1259–1268. doi:10.1093/treephys/tpp058
Stevens MT, Lindroth RL (2005) Induced resistance in the indeterminate growth of aspen (Populus tremuloides). Oecologia 145(2):298–306
Stowe KA (1998) Experimental evolution of resistance in Brassica rapa: correlated response of tolerance in lines selected for glucosinolate content. Evolution 52(3):703–712
Sultan SE (2000) Phenotypic plasticity for plant development, function and life history. Trends Plant Sci 5(12):537–542
Sultan SE (2004) Promising directions in plant phenotypic plasticity. Perspect Plant Ecol Evol Syst 6(4):227–233
Sultan SE (2010) Plant developmental responses to the environment: eco-devo insights. Curr Opin Plant Biol 13(1):96–101
Sultan SE, Bazzaz FA (1993) Phenotypic plasticity in Polygonum persicaria. II. Norms of reaction to soil moisture and the maintenance of genetic diversity. Evolution 47(4):1032–1049
Taiz L, Zeiger E (2010) Plant physiology, 5th edn. Sinauer associates, Sunderland
Textor S, Gershenzon J (2009) Herbivore induction of the glucosinolate-myrosinase defense system: major trends, biochemical bases and ecological significance. Phytochem Rev 8(1):149–170
Waller DM, Dole J, Bersch AJ (2008) Effects of stress and phenotypic variation on inbreeding depression in Brassica rapa. Evolution 62(4):917–931
Wathelet J-P, Iori R, Leoni O, Rollin P, Quinsac A, Palmieri S (2004) Guidelines for glucosinolate analysis in green tissues used for biofumigation. Agroindustria 3(3):257–266
Williams PH, Hill CB (1986) Rapid-cycling populations of Brassica. Science 232(4756):1385–1389
Windig JJ, de Kovel CGF, de Jong G (2004) Genetics and mechanics of plasticity. In: DeWitt TJ, Scheiner SM (eds) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, New York
Zolla G, Heimer YM, Barak S (2010) Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J Exp Bot 61(1):211–224
Zvereva EL, Kozlov MV, Niemelä P, Haukioja E (1997) Delayed induced resistance and increase in leaf fluctuating asymmetry as responses of Salix borealis to insect herbivory. Oecologia 109(3):368–373
Acknowledgments
The authors wish to thank Mrs. Birgitte B. Rasmussen for technical assistance in glucosinolate analysis, George Ellmore for comments on a previous draft, and Jan A. Pechenik for discussions during experimental design. Adam Steinbrenner wishes to thank the Paula Frazier Poskitt Memorial Scholarship and the Astronaut Scholarship Foundation for scholarship support at Tufts University. This project was supported by the Neubauer Scholars Program, Tufts University Summer Scholars, and Torben og Alice Frimodts Fond.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Steinbrenner, A.D., Agerbirk, N., Orians, C.M. et al. Transient abiotic stresses lead to latent defense and reproductive responses over the Brassica rapa life cycle. Chemoecology 22, 239–250 (2012). https://doi.org/10.1007/s00049-012-0113-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-012-0113-y