Abstract
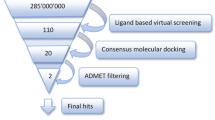
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with yet no effective drug treatment; although several anticholinesterases are being used to offer relief from the symptoms of the disease. Recent studies have indicated that over-activation of cyclooxygenase-2 (COX-2) and matrix metalloproteinase-8 (MMP-8) may cause neuronal death in the brain of AD subjects, suggesting that inhibition of COX-2 and MMP-8 may be of therapeutic value in the management of AD. Therefore, it is important and rational to investigate new agents with anticholinesterase, COX-2 and MMP-8 inhibitory activities. In this study, molecular docking study was performed with earlier identified anticholinesterase alkaloids to search for compounds with high affinity for COX-2 and MMP-8. Molecular docking was done using Blind Docking Server while ligand-protein molecular interaction of compounds with remarkable inhibitory characteristics against COX-2 and MMP-8 were viewed with PyMOL. Alkaloids with high binding affinity and remarkable binding interaction with the target proteins were subjected to drug likeness investigation based on absorption-distribution-metabolism-excretion (ADME) properties using the Swiss online ADME web tool. Nine alkaloids (haloxysterol A, haloxysterol B, haloxysterol C, haloxysterol D, sarcodine, isosarcodine, axillaridine A, sarsalignenone and voacangine hydroxyindolenine) showed high affinities for both COX-2 and MMP-8. Thus, this in silico study identified 9 orally drugable, anticholinesterase alkaloids with COX-2 and MMP-8 multi-target activities that could be studied further as agents against AD.










Similar content being viewed by others
References
Ahmed F, Ghalib RM, Sasikala P, Ahmed KM (2013) Cholinesterase inhibitors from botanicals. Pharm Rev 7(14):121
Ahmed E, Nawaz SA, Malik A, Choudhary MI (2006) Isolation and cholinesterase-inhibition studies of sterols from Haloxylon recurvum. Bioorg Med Chem Lett 16:573–580
Aisen PS (2002) Evaluation of selective COX-2 inhibitors for the treatment of Alzheimer’s disease. J Pain Symptom Manag 23(4):S35–S40
Andrade MT, Lima JA, Pinto AC, Rezende CM, Carvalho MP, Epifanio RA (2005) Indole alkaloids from Tabernaemontana australis (Müell. Arg) Miers that inhibit acetylcholinesterase enzyme. Bioorg Med Chem 13(12):4092–4095
Ata A, Iverson CD, Kalhari KS, Akhter S, Betteridge J, Meshkatalsadat MH, Orhan I, Sener B (2010) Triterpenoidal alkaloids from Buxus hyrcana and their enzyme inhibitory, anti-fungal and anti-leishmanial activities. Phytochemistry 71:1780–1786
Atta-ur-Rahman ZU, Khalid A, Anjum S, Khan MR, Choudhary MI (2002) Pregnane-type steroidal alkaloids of Sarcococca saligna: A new class of cholinesterase inhibitors. Helv Chim Acta 85:678–688
Camps P, Formosa X, Galdeano C, Gómez T, Muñoz-Torrero D, Ramírez L, Viayna E, Gómez E, Isambert N, Lavilla R, Badia A (2010) Tacrine-based dual binding site acetylcholinesterase inhibitors as potential disease-modifyinganti-Alzheimer drug candidates. Chem-biol Inter 187(1–3):411–415
Choudhary MI (2001) Bioactive natural products as a potential source of new pharmacophores. A theory of memory. Pure Appl Chem 73(3):555–560
Daina A, Michielin O, Zoete V (2014) ILOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J Chem Inf Model 54(12):3284–3301
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717
Daina A, Zoete V (2016) A BOILED‐Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 11(11):1117–1121
Dall’Acqua S (2013) Plant-derived acetylcholinesterase inhibitory alkaloids for the treatment of Alzheimer disease. Bot: Targets Ther 3:19–28
Elgorashi EE, Stafford GI, Van Staden J (2004) Acetylcholinesterase enzyme inhibitory effects of Amaryllidaceae alkaloids. Planta Med 70(03):260–262
Giovannini MG, Scali C, Biotech S, Bellucci A, Pepeu G (2003) Experimental brain inflammation and neurodegeneration as model of Alzheimer’s disease: Protective effects of selective COX-2 inhibitors. Int J Immunopathol Pharm 16(2):19–28
Gleeson M, Hersey A, Hannongbua S (2011) In silico ADME Models: A General Assessment of their Utility in Drug Discovery Applications. Curr Top Med Chem 11(4):358–381
Goodwin JT (2005) In silico Predictions of Blood-Brain Barrier Penetration: Considerations to “Keep in Mind.”. J Pharm Exp Ther 315(2):477–483
Hoerr R, Noeldner M (2002) Ensaculin (KA-672. HCl): A Multitransmitter Approach to Dementia Treatment. CNS Drug Rev 8:143–158
Howes MJ, Houghton PJ (2009) Acetylcholinesterase inhibitors of natural origin. Int J Biomed Pharm Sci 3(SI1):67–86
Hu X, Dong W, Cui Z, Gao C, Yu Z, Yuan Q, Min Z (2018) In silico identification of AChE and PARP-1 dual-targeted inhibitors of Alzheimer’ s disease. J Mol Model 24:150–158
Hussain G, Rasul A, Anwar H, Aziz N, Razzaq A, Wei W, Ali M, Li J, Li X (2018) Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int J Bio Sci 14(3):341
Karczmar A (1998) Invited review. Anticholinesterases: Dramatic aspects of their use and misuse. Neurochem Int 32(5-6):401–411
Kavanagh S, Gaudig M, Van Baelen B, Adami M, Delgado A, Guzman C, Jedenius E, Schäuble B (2011) Galantamine and behavior in Alzheimer disease: analysis of four trials. Acta Neurol Scand 124:302–308
Khalid A, Zaheer-Ul-Haq, Ghayur MN, Feroz F, Atta-Ur-Rahman Gilani AH, Choudhary MI (2004) Cholinesterase inhibitory and spasmolytic potential of steroidal alkaloids. J Steroid Biochem Mol Biol 92(5):477–484
Kim MH, Kim SH, Yang WM (2014) Mechanisms of action of phytochemicals from medicinal herbs in the treatment of Alzheimer’s disease. Planta Med 80:1249–1258
Kim DK, Lee KT, Baek NI, Kim SH, Park HW, Lim JP, Shin TY, Eom DO, Yang JH, Eun JS (2004) Acetylcholinesterase inhibitors from the aerial parts of Corydalis speciosa. Arch Pharmacal Res 27(11):1127
Krátký M, Štěpánková Š, Vorčáková K, Švarcová M, Vinšová J, Decker M (2016) Novel cholinesterase inhibitors based on O-aromatic N, N-disubstituted carbamates and thiocarbamates. Molecules 21(2):191
Kumar GP, Khanum F (2012) Neuroprotective potential of phytochemicals. Pharmacogn Rev 6(12):81. 6(12):81
Lin H, Li Q, Gu K, Zhu J, Jiang X, Chen Y, Sun H (2017) Design of multi-target agents for the treatment of Alzheimer’s disease based on tacrine. Curr Top Med Chem 17(27):3000–3016
Lipinski CA (2000) Drug-like properties and the causes of poor solubility and poor permeability. J Pharm Toxicol Methods 44(1):235–249
López S, Bastida J, Viladomat F, Codina C (2002) Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci 71(21):2521–9
Mohsen UA (2012) Studies on imidazopyridine derivatives as acetylcholinesterase inhibitors. Clin Exp Health Sci 2(3):119
Möller HJ, Graeber M (1998) The case described by Alois Alzheimer in 1911 Historical and conceptual perspectives based on the clinical record and neurohistological sections. Eur Arch Psychiatry Clin Neurosci 248:111–122
Mukherjee PK, Kumar V, Mal M, Houghton PJ (2007) Acetylcholinesterase inhibitors from plants. Phytomedicine 14(4):289–300
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: An Open chemical toolbox. J Chemin- 3(10):33
Orhan IE, Orhan G, Gurkas E (2011) An Overview on Natural Cholinesterase Inhibitors - A Multi-Targeted Drug Class - and Their Mass Production. Mini-Rev Med Chem 11:836–842
Orhan I, Şener B, Choudhary MI, Khalid A (2004) Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharmacol 91(1):57–60
Pardridge WM (2009) Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimer’s Dement 5(5):427–432
Park CH, Kim SH, Choi W, Lee YJ, Kim JS, Kang SS, Suh YH (1996) Novel anticholinesterase and antiamnesic activities of dehydroevodiamine, a constituent of Evodia rutaecarpa. Planta Med 62(05):405–9
Pasinetti GM, Aisen PS (1998) Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience 87(2):319–24
Paula A, Belén M, Julia M, Paola N, Cavallaro V (2013) Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr Neuropharmacol 11:388–413
Peress N, Perillo E, Zucker S (1995) Localization of tissue inhibitor of matrix metalloproteinases in Alzheimer’s disease and normal brain. J Neuropathol ExpNeurol 54:16–22
Plummer SM, Holloway KA, Manson MM (1999) Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kB activation via the NIK/IKK signalling complex. Oncogene 18:6013–6020
Pochetti G, Montanari R, Gege C, Chevrier C, Taveras AG, Mazza F (2009) Extra binding region induced by non-zinc chelating inhibitors into the S1′ subsite of matrix metalloproteinase 8 (MMP-8). J Med Chem 52:1040–1049
Rasool M, Arif Malik SW, Tul-Ain Q, Jafar TH, Rasool R, Kalsoom A, Ghafoor MA, Sehgal SA, Gauthaman K, Naseer MI, Al-Qahtani MH (2018) In-Silico Characterization and In-vivo Validation of Albiziasaponin-A, Iso-Orientin, and Salvadorin Using a Rat Model of Alzheimer’s Disease. Front Pharm 9:1–15
Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8(2):205–216
Sánchez-Linares I, Pérez-Sánchez H, Cecilia JM, García JM (2012) High-Throughput parallel blind Virtual Screening using BINDSURF. BMC Bioinforma 13(14):S13
Selvaraj C, Tripathi SK, Reddy KK, Singh SK (2011) Tool development for Prediction of pIC 50 values from the IC 50 values-A pIC 50 value calculator. Curr Trends Biotechnol Pharm 1(5):2
Song JH, Yu JT, Tan L (2015) Brain-Derived Neurotrophic Factor in Alzheimer’s Disease: Risk, Mechanisms, and Therapy. Mol Neurobiol 52(3):1477–1493
Sugimoto H, Yamanishi Y, Iimura Y, Kawakami Y (2000) Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr Med Chem 7:303–339
Sultana N, Sultana R (2009) A new lanostane triterpene from skimmia laureola. Z Nat 64(4):459–463
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
ul Haq Z, Uddin R (2011) Structure Based 3D-QSAR Studies on Cholinesterase Inhibitors. In Alzheimer’s Disease Pathogenesis-Core Concepts, Shifting Paradigms and Therapeutic Targets. IntechOpen https://doi.org/10.5772/17891
Veber DF, Johnson SR, Cheng H, Smith BR, Ward KW, Kopple KD (2002) Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J Med Chem 45:2615–2623
Venkatesan R, Ji E, Kim SY (2015) Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: a comprehensive review. BioMed Res Int 2015:814068. https://doi.org/10.1155/2015/814068
Woodling NS, Colas D, Wang Q, Minhas P, Panchal M, Liang X, Mhatre SD, Brown H, Ko N, Zagol-Ikapitte I, van der Hart M (2016) Cyclooxygenase inhibition targets neurons to prevent early behavioural decline in Alzheimer’s disease model mice. Brain 139(7):2063–2081
Yong VW, Power C, Edwards DR (2001) Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2(7):502
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishola, A.A., Adewole, K.E. In silico screening of anticholinesterase alkaloids for cyclooxygenase-2 (COX-2) and matrix metalloproteinase 8 (MMP-8) inhibitory potentials as multi-target inhibitors of Alzheimer’s disease. Med Chem Res 28, 1704–1717 (2019). https://doi.org/10.1007/s00044-019-02407-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02407-4