Abstract
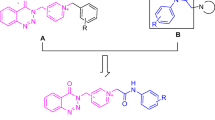
A series of new coumarin/piperazine hybrids were synthesized and evaluated for anticholinesterase activity. Among them, compounds 4d and 4t exhibited potent human acetylcholinesterase (hAChE) inhibitory activity with IC50 values of 2.42 and 9.89 μM, respectively, and 4t displayed highest selectivity toward hAChE over human butyrylcholinesterase (hBChE) by 9.8-fold. In addition, both compounds did not show observed cytotoxicity against SH-SY5Y neuroblastoma cell line at 100 μM. Kinetic analysis in tandem with molecular docking study revealed that these hybrids targeted both catalytic active site (CAS) and peripheral anionic site (PAS) of hAChE. The preliminary results highlighted 4t as an anti-Alzheimer’s disease lead compound worthy of further investigation.





Similar content being viewed by others
References
Barreiro EJ, Camara CA, Verli H, Brazil-Más L, Castro NG, Cintra WM, Aracava Y, Rodrigues CR, Fraga CA (2003) Design, synthesis, and pharmacological profile of novel fused pyrazolo[4,3-d]pyridine and pyrazolo[3,4-b][1,8]naphthyridine isosteres: a new class of potent and selective acetylcholinesterase inhibitors. J Med Chem 46:1144–1152
Bartus RT, Dean RL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414
Bhat MA, Al-Omar MA, Siddiqui N (2013) Antimicrobial activity of Schiff bases of coumarin-incorporated 1,3,4-oxadiazole derivatives: an in vitro evaluation. Med Chem Res 22:4455–4458
Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, Franklin MC, Height JJ (2012) Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem 55:10282–10286
Dandriyal J,Singla R,Kumar M,Jaitak V,(2016) Recent developments of C-4 substituted coumarin derivatives as anticancer agents Eur J Med Chem 119:141–168
Delogu GL, Matos MJ, Fanti M, Era B, Medda R, Pieroni E, Fais A, Kumar A, Pintus F (2016) 2-Phenylbenzofuran derivatives as butyrylcholinesterase inhibitors: synthesis, biological activity and molecular modeling. Bioorg Med Chem Lett 26:2308–2313
Demir Özkay Ü, Can ÖD, Sağlık BN, Turan N (2017) A benzothiazole/piperazine derivative with acetylcholinesterase inhibitory activity: Improvement in streptozotocin-induced cognitive deficits in rats Pharmacol Rep 69:1349–1356
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Feng B, Li X, Xia J, Wu S (2017) Discovery of novel isoflavone derivatives as AChE/BuChE dual-targeted inhibitors: synthesis, biological evaluation and molecular modelling. J Enzy Inhib Med Chem 32:968–977
Furuya T, Kamlet AS, Ritter T (2011) Catalysis for fluorination and trifluoromethylation. Nature 473:470–477
Girisha HR, Narendra Sharath Chandra JN, Boppana S, Malviya M, Sadashiva CT, Rangappa KS (2009) Active site directed docking studies: synthesis and pharmacological evaluation of cis-2,6-dimethyl piperidine sulfonamides as inhibitors of acetylcholinesterase. Eur J Med Chem 44:4057–4062
Hassan MZ, Osman H, Ali MA, Ahsan MJ (2016) Therapeutic potential of coumarins as antiviral agents. Eur J Med Chem 123:236–255
Herrmann N, Chau SA, Kircanski I, Lanctot KL (2011) Current and emerging drug treatment options for Alzheimer’s: disease a systematic review. Drugs 71:2031–2065
Hoerr R, Noeldner M (2002) Ensaculin (KA-672 HCl): a multitransmitter approach to dementia treatment. CNS Drug Rev 8:143–158
Huey R,Morris GM,Olson AJ,Goodsell DS,(2007) A semiempirical free energy force field with charge-based desolvation J Comput Chem 28:1145–1152
Inoue T, Wang F, Moriguchi A, Shirakawa K, Matsuoka N, Goto T (2001) FK960, a novel potential anti-dementia drug, enhances high K+-evoked release of somatostatin from rat hippocampal slices. Brain Res 892:111–117
Jameel E, Umar T, Kumar J, Hoda N (2016) Coumarin: a privileged scaffold for the design and development of antineurodegenerative agents. Chem Biol Drug Des 87:21–38
Li H, Yao Y, Li L (2017) Coumarins as potential antidiabetic agents. J Pharm Pharmacol 69:1253–1264
Li JC,Zhang J,Rodrigues MC,Ding DJ,Longo JP,Azevedo RB,Muehlmann LA,Jiang CS,(2016) Synthesis and evaluation of novel 1,2,3-triazole-based acetylcholinesterase inhibitors with neuroprotective activity Bioorg Med Chem Lett 26:3881–3385
Matsuoka N, Aigner TG (1997) FK960 [N-(4-acetyl-1-piperazinyl)-p-fluorobenzamide monohydrate], a novel potential antidementia drug, improves visual recognition memory in rhesus monkeys: comparison with physostigmine. J Pharmacol Exp Ther 280:1201–1209
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nachon F, Carletti E, Ronco C, trovaslet M, Nicolet Y, Jean L, Renard P (2013) Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl and butyrylcholinesterase. Biochem J 453:393–399
Revankar HM, Bukhari SN, Kumar GB, Qin HL (2017) Coumarins scaffolds as COX inhibitors. Bioorg Chem 71:146–159
Talesa TN (2001) Acetylcholinesterase in Alzheimer’s disease. Mech Ageing Dev 122:1961–1969
van Greunen DG, Cordier W, Nell M, van der Westhuyzen C, Steenkamp V, Panayides JL, Riley DL (2017) Targeting Alzheimer’s disease by investigating previously unexplored chemical space surrounding the cholinesterase inhibitor donepezil. Eur J Med Chem 127:671–690
Weinstock M (2012) Selectivity of cholinesterase inhibition. CNS Drugs 12:307–323
Więckowska A, Więckowski K, Bajda M, Brus B, Sałat K, Czerwińska P, Gobec S, Filipek B, Malawska B (2015) Synthesis of new N-benzylpiperidine derivatives as cholinesterase inhibitors with β-amyloid anti-aggregation properties and beneficial effects on memory in vivo. Bioorg Med Chem 23:2445–2457
Yu YF, Huang YD, Zhang C, Wu XN, Zhou Q, Wu D, Wu Y, Luo HB (2017) Discovery of novel pyrazolopyrimidinone derivatives as phosphodiesterase 9A inhibitors capable of inhibiting butyrylcholinesterase for treatment of Alzheimer’s disease. ACS Chem Neurosci 8:2522–2534
Acknowledgements
This research work was financially supported by the Natural Science Foundation of China (No. 21672082) and Shandong Key Development Project (No. 2016GSF201209).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, J., Jiang, CS. Synthesis and evaluation of coumarin/piperazine hybrids as acetylcholinesterase inhibitors. Med Chem Res 27, 1717–1727 (2018). https://doi.org/10.1007/s00044-018-2185-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2185-x