Abstract
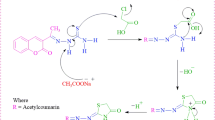
A series of total twenty-one thiazole-coumarin derivatives 7a-u, linked via hydrazine linkage were synthesized through Hantzsch cyclisation. Out of twenty-one derivatives, fourteen derivatives viz. 7b-d, 7g, 7i-k, 7n and 7p-u are the novel derivatives. The structures of the synthesized compounds were established by extensive spectroscopic studies (FTIR, 1H NMR, 13C NMR, 2D NMR, LC-MS) and elemental analysis. The structure of (E)-6-methoxy-3-(1-(2-(4-p-tolylthiazol-2-yl)hydrazono)ethyl)-2H-chromen-2-one (7d) was unambiguously confirmed by X-ray crystallography analysis. Hybrid molecules were evaluated for their potential as anti-tubercular agents against Mycobacterium tuberculosis H37Rv ATCC 25618, and anti-bacterial agents against Eschericia coli, Enterobacter aerogenes, Salmonella typhi, Streptococcus pneumoniae and Staphylococcus aureus. All the compounds displayed considerable potency against all the pathogens with MIC values ranging from 31.25 to 250 μg/mL, therein compounds 7i, 7j, 7k, 7q and 7t displayed superior inhibitory activities compared to standard drugs streptomycin, kanamycin, vancomycin and isoniazid. Molecular docking studies were performed to check the potential as dengue virus NS2B/NS3 serine protease inhibitors, by comparing to standards 4-hydroxypanduratin, panduratin and ethyl 3-(4-(hydroxymethyl)-2-methoxy-5-nitrophenoxy)propanoate with DS of −3.379, −3.189 and −3.381, respectively. All the compounds were found to exhibit potency against the DENV virus. In particular, compound 7c (DS –5.141) and 7l (DS –3.894) were found to be even better than the standards followed by compounds 7j (DS –3.113) and 7q (DS –3.561).










Similar content being viewed by others
References
Brown ED, Wright GD (2016) Antibacterial drug discovery in the resistance era. Nature 529(7586):336–343
Centers for Disease Control and Prevention (CDC) (2009) Plan to combat extensively drug-resistant tuberculosis: recommendations of the Federal Tuberculosis Task Force. MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recomm Rep/Cent Dis 58(RR-3):1
Chimenti F, Bizzarri B, Bolasco A, Secci D, Chimenti P, Granese A, Carradori S, D’Ascenzio M, Scaltrito MM, Sisto F (2010) Synthesis and anti‐Helicobacter pylori activity of 4-(coumarin-3-yl) thiazol-2-ylhydrazone derivatives. J Heterocycl Chem 47(6):1269–1274
El-Agrody AM, Abd El-Latif MS, El-Hady NA, Fakery AH, Bedair AH (2001) Heteroaromatization with 4-hydroxycoumarin part II: synthesis of some new pyrano [2, 3-d] pyrimidines,[1, 2, 4] triazolo [1, 5-c] pyrimidines and pyrimido [1, 6-b]-[1, 2, 4] triazine derivatives. Molecules 6(6):519–527
El-Faragy AF (1991) Synthesis and some reactions of 8-tert-Butyl-6-hydroxy-4-methyl coumarin. Egypt J Pharm Sci 32:625–625
Erbel P, Schiering N, D’Arcy A, Renatus M, Kroemer M, Lim SP, Yin Z, Keller TH, Vasudevan SG, Hommel U (2006) Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat Struct Mol Biol 13(4):372–373
Frimayanti N, Chee CF, Zain SM, Rahman NA (2011) Design of new competitive dengue Ns2b/Ns3 protease inhibitors—a computational approach. Int J Mol Sci 12(2):1089–1100
Ghosh S, Indukuri K, Bondalapati S, Saikia AK, Rangan L (2013) Unveiling the mode of action of antibacterial labdane diterpenes from Alpinia nigra (Gaertn.) B. L. Burtt seeds. Eur J Med Chem 66:101–105
Holla BS, Malini KV, Rao BS, Sarojini BK, Kumari NS (2003) Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and antiinflammatory agents. Eur J Med Chem 38(3):313–318
Hummelova J, Rondevaldova J, Balastikova A, Lapcik O, Kokoska L (2014) The relationship between structure and in vitro antibacterial activity of selected isoflavones and their metabolites with special focus on antistaphylococcal effect of demethyltexasin. Lett Appl Microbiol 60:242–247
Kadir SL, Yaakob H, Zulkifli RM (2013) Potential anti-dengue medicinal plants: a review. J Nat Med 67(4):677–689
Khan Yusufzai S, Osman H, Khan MS, Mohamad S, Sulaiman O, Parumasivam T, Gansau JA, Johansah N (2017) Design, characterization, in vitro antibacterial, antitubercular evaluation and structure–activity relationships of new hydrazinyl thiazolyl coumarin derivatives Med Chem Res 26(6):1139–1148
Kitchen DB, Decornez H, Furr JR, Bajorath J (2004) Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov 3(11):935–949
Kubmarawa D, Khan ME, Punah AM, Hassan M (2008) Phytochemical Screening and antibacterial activity of extracts from Pakia Clapperotoniana keay against human pathogenic bacteria. J Med Plants Res 2(12):352–355
Kumar HN, Parumasivam T, Jumaat F, Ibrahim P, Asmawi MZ, Sadikun A (2014) Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med Chem Res 23(1):269–279
Lengauer T, Rarey M (1996) Computational methods for biomolecular docking. Curr Opin Struct Biol 6(3):402–406
Lee YK, Tan SK, Wahab HA, Rohana Y (2007) Nonsubstrate based inhibitors of dengue virus serine protease: a molecular docking approach to study binding interactions between protease and inhibitors. Asia Pac J Mol Biol Biotechnol 15(2):53–59
Li J, Lim SP, Beer D, Patel V, Wen D, Tumanut C, Tully DC, Williams JA, Jiricek J, Priestle JP, Harris JL (2005) Functional profiling of recombinant NS3 proteases from all four serotypes of dengue virus using tetrapeptide and octapeptide substrate libraries. J Biol Chem 280(31):28766–28774
Liu W, Tao C, Tang L, Li J, Jin Y, Zhao Y, Hu H (2011) A convenient and efficient synthesis of heteroaromatic hydrazone derivatives via cyclization of thiosemicarbazone with ω‐bromoacetophenone. J Heterocycl Chem 48(2):361–364
Ma C, Case RJ, Wang Y, Zhang HJ, Tan GT, Van Hung N, Cuong NM, Franzblau SG, Soejarto DD, Fong HH, Pauli GF (2005) Anti-tuberculosis constituents from the stem bark of Micromelum hirsutum. Planta Med 71(3):261–267
Mahato S, Singh A, Rangan L, Jana CK (2016) Synthesis, In silico studies and In vitro evaluation for antioxidant and antibacterial properties of diarylmethylamines: a novel class of structurally simple and highly potent pharmacophore. Eur J Pharm Sci 88:202–209
Manolov I, Danchev ND (1995) Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarin derivatives. Eur J Med Chem 30(6):531–535
Morens DM, Fauci AS (2008) Dengue and hemorrhagic fever: a potential threat to public health in the United States. J Am Med Assoc 299(2):214–216
Nofal ZM, El-Zahar MI, Abd El-Karim SS (2000) Novel coumarin derivatives with expected biological activity. Molecules 5(2):99–113
Patonay T, Litkei GY, Bognar R, Erdei J, Miszti C (1984) Synthesis, antibacterial and antifungal activity of 4-hydroxycoumarin derivatives, analogues of novobiocin. Die Pharm 39(2):84–91
Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6(1):29–40
Raev LD, Voinova E, Ivanov IC, Popov D (1990) Antitumor activity of some coumarin derivatives. Die Pharm 45(9):696–701
Shaker RM (1996) Synthesis and reactions of some new 4H-pyrano [3, 2-c] benzopyran-5-one derivatives and their potential biological activities. Die Pharm 51(3):148–151
Sharma P, Pritmani S (1999) Synthesis, characterization and antimicrobial studies of some novel 3-arylazo-7-hydroxy-4-methylcoumarins. Indian J Chem B 38:1139–1142
Van Hell AJ, Crommelin DJ, Hennink WE, Mastrobattista E (2009) Stabilization of peptide vesicles by introducing inter-peptide disulfide bonds. Pharm Res 26(9):2186–2193
Walther B, Vis P, Taylor A (2008) Lipophilicity of metabolites and its role in biotransformation. In: Pliska V, Testa B, van de Waterbeemd H (eds) Lipophilicity in drug action and toxicology, vol 4, Wiley-VCH
Xie L, Takeuchi Y, Cosentino LM, Lee KH (1999) Anti-AIDS agents. 37. 1 synthesis and structure−activity relationships of (3 ‘R, 4 ‘R)-(+)-cis-Khellactone derivatives as novel potent anti-HIV agents. J Med Chem 42(14):2662–2672
Yoneyama H, Katsumata R (2006) Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci Biotechnol Biochem 70(5):1060–1075
Zhuravel I, Kovalenko S, Vlasov S, Chernykh V (2005) Solution-phase synthesis of a combinatorial library of 3-[4-(Coumarin-3-yl)-1, 3-thiazol-2-ylcarbamoyl] propanoic acid amides. Molecules 10(2):444–456
Acknowledgements
We would like to thank the Malaysian Government and Universiti Sains Malaysia (USM) for providing necessary research facilities and Research University Grant (FRGS 203/PKIMIA/6711462). Samina Khan Yusufzai (SKY) thanks the Institute of Postgraduate Studies (IPS), USM for the Graduate Assistance fellowship support. SKY expresses the gratitude to the School of Biological Sciences, USM and Faculty of Science and Natural Resources, Universiti Malaysia Sabah for providing facilities for biological studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yusufzai, S.K., Osman, H., Khan, M.S. et al. Synthesis, X-ray crystallographic study, pharmacology and docking of hydrazinyl thiazolyl coumarins as dengue virus NS2B/NS3 serine protease inhibitors. Med Chem Res 27, 1647–1665 (2018). https://doi.org/10.1007/s00044-018-2179-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2179-8