Abstract
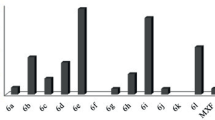
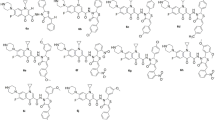
A series of 2- or 3-(4,5-dihydro-1H-imidazol-2-yl)-1H-indole derivatives were synthesized, characterized, and evaluated for their in vitro antibacterial and antifungal activities. Additionally, the synthesized compounds were docked into the II DNA gyrase B active site, and their predicted binding modes were inspected. Inhibitory activity were tested against two species of Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa), two species of Gram-positive bacteria (Staphylococcus aureus, Listeria monocytogenes) and two fungi (Candida albicans, Aspergillus niger) using the broth microdilution method. The fluorine-substituted 2-(2-imidazolyl)indole 2b was found to be the most potent antibacterial compound against E. coli and S. aureus strains (MIC value 80 μg/mL). Compounds showed better activity against Gram-positive bacteria compared to Gram-negative bacteria. The docking results predicted that the imidazoline-indole hybrid moiety bind to the active site protein ATP-binding pocket from E. coli and S. aureus with good interaction energy scores. The significant loss of antibacterial activity for some imidazoline-indole analogs could be attributed to several nonoptimal enzyme interactions, including poor hydrogen bonds provided by Asp73 (E. coli gyrase numbering) or Asp81 (S. aureus gyrase numbering) and an associated water molecule.



Similar content being viewed by others
References
Azam MA, Thathan J, Jubie S (2015) Dual targeting DNA gyrase B (GyrB) and topoisomerse IV (ParE) inhibitors: a review. Bioorg Chem 62:41–63
Bansal S, Kumar S, Aggarwal V, Joseph A (2014) Design, synthesis, docking study & antibacterial evaluation of 1,3-diarylpyrazolyl substituted indolin-2-ones. Indo Global J Pharm Sci 4:1–7
Bisacchi GS, Manchester JI (2015) A new-class antibacterial-almost. Lessons in drug discovery and development: a critical analysis of more than 50 years of effort toward ATPase inhibitors of DNA gyrase and topoisomerase IV. ACS Infect Dis 1:4–41
Brvar M, Perdih A, Renko M, Anderluh G, Turk D, Solmajer T (2012) Structure-based discovery of substituted 4,5’-bithiazoles as novel DNA gyrase inhibitors. J Med Chem 55:6413–6426
Crouch RD (2009) Synthetic routes toward 2-substituted-2-imidazolines. Tetrahedron 65:2387–2397
Dash P, Kudav DP, Parihar JA (2004) Thioacetamide catalyzed transformation of nitriles to 2-substituted imidazolines. J Chem Res 7:490–491
Desroy N, Denis A, Oliveira C, Atamanyuk D, Briet S, Faivre F, LeFralliec G, Bonvin Y, Oxoby M, Escaich S, Floquet S, Drocourt E, Vongsouthi V, Durant L, Moreau F, Verhey TB, Lee T-W, Junop MS, Gerusz V (2013) Novel HldE-K inhibitors leading to attenuated gram negative bacterial virulence. J Med Chem 56:1418–1430
Fonquerna S, Miralpeix M, Pagès L, Puig C, Cardús A, Antón F, Cárdenas A, Vilella D, Aparici M, Calaf E, Prieto J, Gras J, Huerta JM, Warrellow G, Beleta J, Ryder H (2004) Synthesis and structure-activity relationships of novel Histamine H1 antagonists: Indolylpiperidinyl benzoic acid derivatives. J Med Chem 47:6326–6337
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford CT
Han L, Du DM (2009) Recent advances in the synthesis of 2-imidazolines and their applications in homogeneous catalysis. Adv Synth Catal 351:489–519
Joshi KC, Joshi R (2000) Fluorinated indole derivatives: synthetic and biomedicinal aspects. J Indian Chem Soc 77:627–634
Koenig SG, Dankwardt JW, Liu Y, Zhao H, Singh SP (2010) A ligand-free, copper-catalyzed cascade sequence to indole-2-carboxylic esters. Tetrahedron Lett 51:6549–6551
Kou X, Zhao M, Qiao X, Zhu Y, Tong X, Shen Z (2013) Copper-catalyzed aromatic C-H bond cyanation by C-CN bond cleavage of inert acetonitrile. Chem Eur J 19:16880–16886
KumarMittal A, Singh D, Tripathi S (2011) Synthesis and antifungal activity of some new fluorinated 1-[2-hydroxyethyl]-3-ethoxycarbonyl-5-oxadiazolyl/triazolyl/pyrrolyl-aminocarbonylmethoxy-2-methylbenz[g]indoles. J Chem Bio Phy Sci 1:141–152
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51:2778–2786
Lee JH, Kim YG, Cho MH, Kim JA, Lee J (2012) 7-fluoroindole as an antivirulence compound against Pseudomonas aeruginosa. FEMS Microbiol Lett 329:36–44
Lei L, Hui-Zhu Y, Zhao-Hai Q, Xiao-Jing Y, Yu-Mei X, Nan L, Bin F (2011) Synthesis and fungicidal activity of thiazoline derivatives containing halogenated indolyl moiety. Phosphorus Sulfur Silicon Relat Elem 186:1790–1800
Lewis RJ, Singh OM, Smith CV, Skarzynski T, Maxwell A, Wonacott AJ, Wigley DB (1996) The nature of inhibition of DNA gyrase by the coumarins and the cyclothialidines revealed by X-ray crystallography EMBO J 15:1412–1420
Morales-Ríos MS, García-Vázquez B, Florán-Garduño B, Serrano-Alva MT (2015) Preparation of serotonin reuptake inhibitor for treating central nervous system disorders. Mex Pat Appl MX 2014011573 A 20150310.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. J Comput Chem 16:2785–2791
NCCLS (2003) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA
Nishiyama T, Hatae N, Yoshimura T, Takaki S, Abe T, Ishikura M, Hibino S, Choshi T (2016) 1c Concise synthesis of carbazole-1,4-quinones and evaluation of their antiproliferative activity against HCT-116 and HL-60 cells. Eu J Med Chem 121:561–577
Oblak M, Grdadolnik SG, Kotnik M, Jerala R, Filipic M, Solmajer T (2005) In silico fragment-based discovery of indolin-2-one analogues as potent DNA gyrase inhibitors. Bioorg Med Chem Lett 15:5207–5210
Oblak M, Miha M, Solmajer T (2007) Discovery and development of ATPase inhibitors of DNA gyrase as antibacterial agents. Curr Med Chem 14:2033–2047
Panchal RG, Ulrich RL, Lane D, Butler MM, Houseweart C, Opperman T, Williams JD, Peet NP, Moir DT, Nguyen T, Gussio R, Bowlin T, Bavari S (2009) Novel broad-spectrum Bis-(imidazolinylindole) derivatives with potent antibacterial activities against antibiotic-resistant strains. Antimicrob Agents Chemother 53:4283–4291
Pérez-Alvarez V, Morales-Ríos MS, Hong E, Joseph-Nathan P (1997) Synthesis of 3-amino-2-(3-indolyl)propanol and propanoate derivatives and preliminary cardiovascular evaluation in rats. J Pharm Pharmacol 49:246–252
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Rivera-Becerril E, Joseph-Nathan P, Pérez-Alvarez VM, Morales-Ríos MS (2008) Synthesis and biological evaluation of (-)- and (+)-debromoflustramine B and its analogues as selective butyrylcholinesterase inhibitors. J Med Chem 51:5271–5284
Sawa N (1968) Nippon Kagaku Zasshi 89:780. (1969) Chem Abstr 70:19983
Servi S, Genc M, Gür G, Koca M (2005) The synthesis and antimicrobial activity of some new methyl N-arylthiocarbamates, dimethyl N-aryldithiocarbonimidates and 2-arylamino-2-imidazolines. Eu J Med Chem 40:687–693
Smart BE (2001) Fluorine substituent effects (on bioactivity). J Fluor Chem 109:3–11
Spadoni G, Diamantini G, Bedini A, Tarzia G, Vacondio F, Silva C, Rivara M, Mor M, Plazzi PV, Zusso M, Franceschini D, Giusti P (2006) Synthesis, antioxidant activity and structure–activity relationships for a new series of 2-(N-acylaminoethyl)indoles with melatonin-like cytoprotective activity. J Pineal Res 40:259–269
Spartan 14 (2013) Spartan’14 for Windows, macintosh, and linux. Wavefunction Inc., Irvine. http://downloads.wavefun.com/Spartan14Manual.pdf.
Tejpal-Singh C, Poonam K, Nutan S, Sunita B (2016) Strategic synthesis and in vitro antimicrobial evaluation of novel difluoromethylated 1-(1, 3-diphenyl-1H-pyrazol-4-yl)-3, 3-difluoro-1, 3-dihydro-indol-2-ones. Med Chem Res 25:2335–2348
Trott O, Olson AJ (2010) Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
WHO (World Health Organization) Antimicrobial resistance, Global Report on Surveillance (2014). http://www.who.int/drugresistance/documents/surveillance-report/en/ Accessed 2 December 2016
Wysong V, Whithe HC (1968) Substituted imidazolinyl índoles. US Patent 3,586,695.
Zhang J, Yang Q, Cross JB, Romero JA, Poutsiaka KM, Epie F, Bevan D, Wang B, Zhang Y, Chavan A, Zhang X, Moy T, Daniel A, Nguyen K, Chamberlain B, Carter N, Shotwell J, Silverman J, Metcalf CA, Ryan D, Lippa B, Dolle RE (2015) Discovery of azaindole ureas as a novel class of bacterial Gyrase B inhibitors. J Med Chem 58:8503–8512
Zhou Y, Wang J, Gu Z, Wang S, Zhu W, Aceña JL, Soloshonok VA, Izawa K, Liu H (2016) Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II−III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem Rev 116:422–518
Zidar N, Macut H, Tomašič T, Brvar M, Montalvão S, Tammela P, Solmajer T, Peterlin Mašič L, Ilaš J, Kikelj D (2015) N-phenyl-4,5-dibromopyrrolamides and N-phenylindolamides as ATP competitive DNA gyrase B inhibitors: design, synthesis, and evaluation. J Med Chem 58:6179–6194
Zoidis G, Giannakopoulou, Stevaert A, Frakolaki E, Myrianthopoulos V, Fytas G, Mavromara P, Mikros E, Bartenschlager R, Vassilaki N, Naesens L (2016) Novel indole–flutimide heterocycles with activity against influenza PA endonuclease and hepatitis C virus. Med Chem Comm 7:447–456
Acknowledgements
The present work is partially supported by CONACYT grant 179187 and Programa de Fortalecimiento Académico del Posgrado de Alta Calidad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Mendoza-Figueroa, H.L., Serrano-Alva, M.T., Aparicio-Ozores, G. et al. Synthesis, antimicrobial activity, and molecular docking study of fluorine-substituted indole-based imidazolines. Med Chem Res 27, 1624–1633 (2018). https://doi.org/10.1007/s00044-018-2177-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2177-x