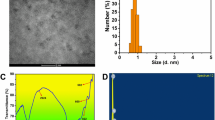
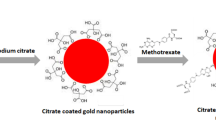
Abstract
Methotrexate (MTX) is a folic acid derivative that is used for the treatment of different malignancies, whose applications have become limited by its toxic dose-related side effect and the possible initiation of drug resistance. The aim of the present research is to study the enhancement of the cellular cytotoxicity of MTX as an anticancer drug by conjugation to the synthesized polymer dots’ (Pdots) surface. This conjugation was developed covalently by carbodiimide chemistry and characterized with Fourier transform infrared spectroscopy (FT-IR), dynamic light scattering (DLS), and UV–Vis spectroscopy. As follows, antineoplastic effect of these synthesized Pdot-MTX nanoconjugates against human breast cancer, Michigan Cancer Foundation-7 (MCF-7), and human cervical (HeLa) cancer cells was investigated and compared with bare MTX and Pdots. The half maximal inhibitory concentration (IC50) of the Pdot-MTX nanoconjugates and the free MTX molecules was obtained 20 and 30 µg mL−1, respectively in the HeLa cells; whereas, these values were about 15 and 25 µg mL−1 for the Pdot-MTX nanoconjugates and the free MTX in the MCF-7 cells. In fact, the conjugation of Pdots with singular properties to MTX enhanced the cellular cytotoxicity of MTX through folate receptors (FRs)-mediated endocytosis that circumvents the efflux functions of cancerous cells. Accordingly, conjugated Pdots with MTX might offer superior hopes for cancer therapeutics and diagnosis as a novel application in future that is of great importance in the fields of drug discovery and chemical biology.











Similar content being viewed by others
References
Abdulrahman O (2012) Submicrometre-scale polyaniline colloidal spheres: photopolymerization preparation using fluorescent carbon nitride dots as a photocatalyst. Catal Sci Technol 2:711–714
Banerjee D, Mayer-Kuckuk P, Capiaux G, Budak-Alpdogan T, Gorlick R, Bertino JR (2002) Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim Biophys Acta 1587:164–173
Barar J, Kafil V, Majd MH, Barzegari A, Khani S, Johari-Ahar M, Asgari D, Cokous G, Omidi Y (2015) Multifunctional mitoxantrone-conjugated magnetic nanosystem for targeted therapy of folate receptor-overexpressing malignant cells. J Nanobiotechnol 13:26
Barar J, Omidi Y (2013) Dysregulated pH in tumor microenvironment checkmates cancer therapy. BioImpacts 3:149–162
Barar J, Omidi Y (2014) Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. BioImpacts 4:3–14
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49:6726–6744
Bawendi MG, Steigerwald ML, Brus LE (1990) The quantum mechanics of larger semiconductor clusters (“ quantum dots. Annu Rev Phys Chem 41:477–496
Biju V, Mundayoor S, Omkumar RV, Anas A, Ishikawa M (2010) Bioconjugated quantum dots for cancer research: present status, prospects and remaining issues. Biotechnol Adv 28:199–213
Brus L (1991) Quantum crystallites and nonlinear optics. Appl Phys A 53:465–474
Byers RJ, Hitchman ER (2011) Quantum dots brighten biological imaging. Prog Histochem Cytochem 45:201–237
Chan Y-H, Jin Y, Wu C, Chiu DT (2011a) Copper (II) and iron (II) ion sensing with semiconducting polymer dots. Chem Commun 47:2820–2822
Chan Y-H, Wu C, Ye F, Jin Y, Smith PB, Chiu DT (2011b) Development of ultrabright semiconducting polymer dots for ratiometric pH sensing. Anal Chem 83:1448–1455
Chen Y-H, Tsai C-Y, Huang P-Y, Chang M-Y, Cheng P-C, Chou C-H, Chen D-H, Wang C-R, Shiau A-L, Wu C-L (2007) Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol Pharm 4:713–722
Chittasupho C, Jaturanpinyo M, Mangmool S (2013) Pectin nanoparticle enhances cytotoxicity of methotrexate against hepG2 cells. Drug Deliv 20:1–9
Doane TL, Burda C (2012) The unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapy. Chem Soc Rev 41:2885–2911
Ekimov A, Efros AL, Onushchenko A (1985) Quantum size effect in semiconductor microcrystals. Solid State Commun 56:921–924
Erogbogbo F, Yong K-T, Hu R, Law W-C, Ding H, Chang C-W, Prasad PN, Swihart MT (2010) Biocompatible magnetofluorescent probes: luminescent silicon quantum dots coupled with superparamagnetic iron (III) oxide. ACS Nano 4:5131–5138
Fletcher JI, Haber M, Henderson MJ, Norris MD (2010) ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer 10:147–156
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer 2:48–58
Henglein A (1989) Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem Rev 89:1861–1873
Hildebrandt N (2011) Biofunctional quantum dots: controlled conjugation for multiplexed biosensors. ACS Nano 5:5286–5290
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG (2013) Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13:714–726
Hoshino A, Fujioka K, Oku T, Suga M, Sasaki YF, Ohta T, Yasuhara M, Suzuki K, Yamamoto K (2004) Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett 4:2163–2169
Hsu PC, Chang HT (2012) Synthesis of high-quality carbon nanodots from hydrophilic compounds: role of functional groups. Chem Commun 48:3984–3986
Issarachot O, Suksiriworapong J, Takano M, Yumoto R, Junyaprasert VB (2014) Folic acid-modified methotrexate-conjugated PEGylated poly (ε-caprolactone) nanoparticles for targeted delivery. J Nanopart Res 16:1–15
Jacobsen NR, Moller P, Jensen KA, Vogel U, Ladefoged O, Loft S, Wallin H (2009) Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/-mice. Part Fibre Toxicol 6:2
Ji J, Wu D, Liu L, Chen J, Xu Y (2012) Preparation, evaluation, and in vitro release of folic acid conjugated O-carboxymethyl chitosan nanoparticles loaded with methotrexate. J Appl Polym Sci 125:E208–E215
Jiang J, He Y, Li S, Cui H (2012) Amino acids as the source for producing carbon nanodots: microwave assisted one-step synthesis, intrinsic photoluminescence property and intense chemiluminescence enhancement. Chem Commun 48:9634–9636
Johari-Ahar M, Barar J, Alizadeh AM, Davaran S, Omidi Y, Rashidi M-R (2016) Methotrexate-conjugated quantum dots: synthesis, characterisation and cytotoxicity in drug resistant cancer cells. J Drug Target 24:120–133
Johari-Ahar M, Rashidi M-R, Barar J, Aghaie M, Mohammadnejad D, Ramazani A, Karami P, Coukos G, Omidi Y (2015) An ultra-sensitive impedimetric immunosensor for detection of the serum oncomarker CA-125 in ovarian cancer patients. Nanoscale 7:3768–3779
Kaur V, Garg T, Rath G, Goyal AK (2014) Therapeutic potential of nanocarrier for overcoming to P-glycoprotein. J Drug Target 22:859–870
Kohler N, Sun C, Wang J, Zhang M (2005) Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir 21:8858–8864
Kruh GD, Belinsky MG (2003) The MRP family of drug efflux pumps. Oncogene 22:7537–7552
Kumar S, Rai AK, Singh V, Rai S (2005) Vibrational spectrum of glycine molecule. Spectrochim Acta Mol Biomol Spectrosc 61:2741–2746
Lai T, Zheng E, Chen L, Wang X, Kong L, You C, Ruan Y, Weng X (2013) Hybrid carbon source for producing nitrogen-doped polymer nanodots: one-pot hydrothermal synthesis, fluorescence enhancement and highly selective detection of Fe (III). Nanoscale 5:8015–8021
Li M, Neoh K-G, Wang R, Zong B-Y, Tan JY, Kang E-T (2013) Methotrexate-conjugated and hyperbranched polyglycerol-grafted Fe3O4 magnetic nanoparticles for targeted anticancer effects. Eur J Pharm Sci 48:111–120
Liu H, Ye T, Mao C (2007) Fluorescent carbon nanoparticles derived from candle soot. Angew Chem Int Ed 46:6473–6475
Liu S, Tian J, Wang L, Luo Y, Sun X (2012) A general strategy for the production of photoluminescent carbon nitride dots from organic amines and their application as novel peroxidase-like catalysts for colorimetric detection of H2O2 and glucose. RSC Adv 2:411–413
Liu S, Tian J, Wang L, Luo Y, Zhai J, Sun X (2011a) Preparation of photoluminescent carbon nitride dots from CCl4 and 1, 2-ethylenediamine: a heat-treatment-based strategy. J Mater Chem 21:11726–11729
Liu S, Wang L, Tian J, Zhai J, Luo Y, Lu W, Sun X (2011b) Acid-driven, microwave-assisted production of photoluminescent carbon nitride dots from N, N-dimethylformamide. RSC Adv 1:951–953
Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG (2008) Compact biocompatible quantum dots functionalized for cellular imaging. JACS 130:1274–1284
Majd MH, Asgari D, Barar J, Valizadeh H, Kafil V, Abadpour A, Moumivand E, Mojarrad JS, Rashidi MR, Coukos G (2013) Tamoxifen loaded folic acid armed PEGylated magnetic nanoparticles for targeted imaging and therapy of cancer. Colloids Surf B 106:117–125
Mamot C, Ritschard R, Wicki A, Küng W, Schuller J, Herrmann R, Rochlitz C (2012) Immunoliposomal delivery of doxorubicin can overcome multidrug resistance mechanisms in EGFR-overexpressing tumor cells. J Drug Target 20:422–432
Mashinchian O, Johari-Ahar M, Ghaemi B, Rashidi M, Barar J, Omidi Y (2014) Impacts of quantum dots in molecular detection and bioimaging of cancer. BioImpacts 4:149–166
Matthaiou E-I, Barar J, Sandaltzopoulos R, Li C, Coukos G, Omidi Y (2014) Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomed 9:1855
Messmann R, Allegra C (2001) Antifolates. Cancer chemotherapy and biotherapy. Lippincott Williams & Wilkins, Philadelphia, p 139–184
Nogueira DR, Tavano L, Mitjans M, Pérez L, Infante MR, Vinardell MP (2013) In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials 34:2758–2772
Omidi Y, Barar J (2014) Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. BioImpacts 4:55–67
Patil Y, Sadhukha T, Ma L, Panyam J (2009) Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release 136:21–29
Pandey S, Thakur M, Mewada A, Anjarlekar D, Mishra N, Sharon M (2013) Carbon dots functionalized gold nanorod mediated delivery of doxorubicin: tri-functional nano-worms for drug delivery, photothermal therapy and bioimaging. J Mater Chem B 1:4972–4982
Prasad PN (2004) Introduction to biophotonics. Wiley, Hoboken
Pu K-Y, Li K, Shi J, Liu B (2009) Fluorescent single-molecular core−shell nanospheres of hyperbranched conjugated polyelectrolyte for live-cell imaging. Chem Mater 21:3816–3822
Rahim NAA, McDaniel W, Bardon K, Srinivasan S, Vickerman V, So PT, Moon JH (2009) Conjugated polymer nanoparticles for two-photon imaging of endothelial cells in a tissue model. Adv Mater 21:3492–3496
Rajagopalan PR, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG (2002) Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc Natl Acad Sci 99:13481–13486
Sarti S, Bordi F (2013) Polymeric hollow micro and nanospheres for biotechnological applications: a focused review. Mater Lett 109:134–139
Seruga B, Ocana A, Tannock IF (2011) Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 8:12–23
Sjöback R, Nygren J, Kubista M (1995) Absorption and fluorescence properties of fluorescein. Spectrochim Acta Mol Biomol Spectrosc 51(6):L7–L21
Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234
Tang J, Kong B, Wu H, Xu M, Wang Y, Wang Y, Zhao D, Zheng G (2013) Carbon nanodots featuring efficient FRET for real-time monitoring of drug delivery and two-photon imaging. Adv Mater 25:6569–6574
Vezmar S, Becker A, Bode U, Jaehde U (2003) Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy 49:92–104
Weller H (1993) Quantized semiconductor particles: a novel state of matter for materials science. Adv Mater 5:88–95
Wu C, Bull B, Christensen K, McNeill J (2009) Ratiometric single-nanoparticle oxygen sensors for biological imaging. Angew Chem Int Ed 48:2741–2745
Wu C, Bull B, Szymanski C, Christensen K, McNeill J (2008) Multicolor conjugated polymer dots for biological fluorescence imaging. ACS Nano 2:2415–2423
Wu C, McNeill J (2008) Swelling-controlled polymer phase and fluorescence properties of polyfluorene nanoparticles. Langmuir 24:5855–5861
Wu C, Peng H, Jiang Y, McNeill J (2006) Energy transfer mediated fluorescence from blended conjugated polymer nanoparticles. J Phys Chem B 110:14148–14154
Wu C, Szymanski C, Cain Z, McNeill J (2007) Conjugated polymer dots for multiphoton fluorescence imaging. JACS 129:12904–12905
Wu PJ, Ou KL, Chen JK, Fang HP, Tzing SH, Lin WX, Chang JY (2014) Methotrexate-conjugated AgInS2/ZnS quantum dots for optical imaging and drug delivery. Mater Lett 128:412–416
Wu Q, Yang Z, Nie Y, Shi Y, Fan D (2014) Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett 347:159–166
Yang S-T, Wang X, Wang H, Lu F, Luo PG, Cao L, Meziani MJ, Liu J-H, Liu Y, Chen M, Huang Y, Sun Y-P (2009) Carbon dots as nontoxic and high-performance fluorescence imaging agents. J Phys Chem C Nanomater Interfaces 113(42):18110–18114
Yang ZC, Li X, Wang J (2011) Intrinsically fluorescent nitrogen-containing carbon nanoparticles synthesized by a hydrothermal process. Carbon 49:5207–5212
Ye F, Wu C, Jin Y, Chan Y-H, Zhang X, Chiu DT (2011) Ratiometric temperature sensing with semiconducting polymer dots. JACS 133:8146–8149
Ye F, Wu C, Jin Y, Wang M, Chan YH, Yu J, Su W, Hayden S, Chiu DT (2012) A compact and highly fluorescent orange-emitting polymer dot for specific subcellular imaging. Chem Commun 48:1778–1780
Yu J, Wu C, Sahu SP, Fernando LP, Szymanski C, McNeill J (2009) Nanoscale 3D tracking with conjugated polymer nanoparticles. JACS 131:18410–18414
Zhang L, Su T, He B, Gu Z (2014) Self-assembly polyrotaxanes nanoparticles as carriers for anticancer drug methotrexate delivery. Nano-Micro Lett 6:108–115
Zhang X, Chibli H, Kong D, Nadeau J (2012) Comparative cytotoxicity of gold–doxorubicin and InP–doxorubicin conjugates. Nanotechnology 23:275103
Zhang X, Chibli H, Mielke R, Nadeau J (2010) Ultrasmall gold−doxorubicin conjugates rapidly kill apoptosis-resistant cancer cells. Bioconjugate Chem 22:235–243
Zhou Y, Shi L, Li Q, Jiang H, Lv G, Zhao J, Wu C, Selke M, Wang X (2010) Imaging and inhibition of multi-drug resistance in cancer cells via specific association with negatively charged CdTe quantum dots. Biomaterials 31:4958–4963
Zrazhevskiy P, Sena M, Gao X (2010) Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem Soc Rev 39:4326–4354
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rafienia, M., Nasirian, V., Mansouri, K. et al. Methotrexate-conjugated to polymer quantum dot for cytotoxicity effect improved against MCF-7 and Hela cells. Med Chem Res 27, 1578–1588 (2018). https://doi.org/10.1007/s00044-018-2173-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2173-1