Abstract
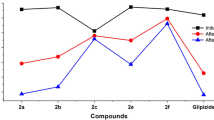
A novel class of sulfonylurea and thiourea derivatives substituted with pyridazine and triazolopyridazine were designed and synthesized. The target compounds were assayed for their effects on the insulin release of alloxan-induced diabetic rats. The results showed that derivatives 4a, 4c, 8a, 11a, and 11b have significant antihyperglycemic effect in an experimental model of diabetes mellitus. No significant differences in cholesterol levels were observed between the diabetic group and diabetic groups that received the test compounds. However, the triglycerides level was reduced significantly by compound 8a when compared with the diabetic group.





Similar content being viewed by others
References
Costantino L, Rastelli G, Vescovini K, Cignarell G, Barlocoo D (2000) Synthesis and aldose reductase inhibitory activity of a new series of benzo[h]cinnolinone derivatives. Farmaco 55:544–552
Coudert P, Duroux E, Bastide P, Couquelet J, Tronche P (1991) Synthesis and evaluation of the aldose reductase inhibitory activity of new diaryl pyridazine-3-ones. J Pharm Belg 46(6):375–380
Deeb A, Said S (1990) Studies on polyazaindenes synthesis of several new condensed pyridazine derivatives collect. Czech Chem Commun 55:2795–2799
Deeb A, Hassaneen M, Kotb M (2005) Pyridazine derivatives and related compounds, part 8: synthesis of different heterocycles from 3-hydrazinopyridazine. Heteroat Chem 12:278–282
DeFronzo RA (1999) Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 131:281–303
DeFronzo RA, Ferrannini E, Koivisto V (1983) New concepts in the pathogenesis and treatment of noninsulin-dependent diabetes mellitus. Am J Med 74:52–81
El-Mariah F, Nassar E, Hosny M, Deeb A (2009) Pyridazine derivatives and related compounds, part 28.1 pyridazinesulfonamides: synthesis and antimicrobial activity phosphorus. Sulfur Silicon 184:92–102
Faidallah HM, Khan KA, Asiri AM (2011) Synthesis and biological evaluation of new 3-trifluoromethylpyrazolesulfonyl-urea and thiourea derivatives as antidiabetic and antimicrobial agents. J Fluor Chem 132(2):75–146
Fassati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28:2077–2080
Haliwell B, Gutteridge JM (1985) Free radicals in biology and medicine, 2nd edn. Clarendon Press, Oxford
Jawale DV, Pratap UR, Rahuja N, Srivastava AK, Mane RA (2012) Synthesis and antihyperglycemic evaluation of new 2,4-thiazolidinediones having biodynamic aryl sulfonylurea moieties. Bioorg Med Chem Lett 22(1):436–439
Kecskemeti V, Bagi Z, Pacher P, Posa I, Koesis E, Koltai MZ (2002) New trends in the development of oral antidiabetic drugs. Curr Med Chem 9:53–71
Radwan S, Bakhite E (1990) Synthese neuer Thieno[2,3-c]pyridazine und verwandter Heterocyclen. Monatshefte Für Chemie 130(9):1117–1128
Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem 19:1350–1356
Schmidt P, Druey J (1954) Heilmittelchemische Studien in der heterocyclischen Reihe. 5. Mitteilung. Pyridazine II. Eine neue Pyridazinsynthese Helv. Chem Acta 37:134–139
Shanmugasundaram KR, Panneerselvam C, Samudram P, Shanmugasundaram ER (1983) Enzyme changes and glucose utilization in diabetic rabbits: the effect of Gymnema sylvestre. J Ethnopharmacol 7:205–234
Trinder P (1969) Enzymatic method of glucose estimation. Ann Clin Biochem 6:24–33
Zhang H-B, Zhang Y-A, Wu G-Z, Zhou J-P, Huang W-L, Hu X-W (2009) Synthesis and biological evaluation of sulfonylurea and thiourea derivatives substituted with benzenesulfonamide groups as potential hypoglycemic agents. Bioorg Med Chem Lett 19(6):1740–1744
Acknowledgments
The authors are grateful for financial support of this work at the Faculty of Science, Zagazig University, through the project “Synthesis and evaluation of some sulfonylureas as novel antidiabetic drugs.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Taken in part from M.Sc. thesis of Sebaey Mahgoub, Zagazig University, 2013.
Rights and permissions
About this article
Cite this article
Deeb, A., El-Eraky, W., El-Awdan, S. et al. Pyridazine and its related compounds. Part 34. Hypoglycemic and hypolipidemic activity of some novel condensed pyridazine sulfonamides. Med Chem Res 23, 34–41 (2014). https://doi.org/10.1007/s00044-013-0605-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0605-5