Abstract
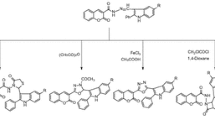
Several mono-Schiff’s bases (3a–i) and bis-Schiff’s bases (5a–f) were synthesized using microwave irradiation technique (3a–h, 5a–c) and by simply grinding at room temperature for a few minutes (3i, 5d–f). All these compounds were characterized by spectroscopic means and elemental analysis. They were screened for anti-inflammatory and anticancer activities (against five human cancer cell lines). Compound 5f exhibited good anti-inflammatory and compounds 3f, 5a–f exhibited good anticancer activity.


Similar content being viewed by others
References
Aydogan F, Ocal N, Turgut Z, Yolacom C (2001) Transformation of aldimines derived from pyrrole-2-carbaldehyde. Synthesis of thiazolidino–fused compounds. Bull Korean Chem Soc 22:476–480
Bawa S, Kumar S (2009) Synthesis of Schiff’s bases of 8-methyltetrazolo [1, 5-a] quinoline as potential anti-inflammatory and antimicrobial agents. Indian J Chem Sect B 48B:142–145
Bhandari SV, Bothara KG, Raut MK, Patil AA, Sarkate AP, Mokale VJ (2008) Design synthesis and evaluation of anti-inflammatory, analgesic and ulcerogenicity studies of novel S-substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as nonulcerogenic derivatives. Bioorg Med Chem 16:1822–1831
Brown JR, DuBios RN (2005) COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol 23:2840–2855
Dios A, Mitchell RA, Aljabari B, Lubetsky J, O’Connor K-A, Liao H, Senter PD, Manogue KR, Lolis E, Metz C, Bucala R, Callaway DJE, Al-Abed Y (2002) Inhibition of MIF bioactivity by rational design of pharmacological inhibitors of MIF tautomerase activity. J Med Chem 45:2410–2416
Geronikaki A, Hadjipavlou-Litina D, Amourgianou M (2003) Novel thiazolyl, thiazolinyl and benzothiazolyl schiff bases as possible lipoxygenase’s inhibitors and anti-inflammatory agents. Farmaco 58:489–495
Grosch S, Maier TJ, Schiffmann S, Geisslinger G (2006) Cyclooxygenase-2 (COX-2)–independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst 98:736–747
Hadjipavlou-Litina DJ, Geronikaki AA (1998) Thiazolyl and benzothiazolyl schiff bases as novel possible lipoxygenase inhibitors and anti-inflammatory agents. Synthesis and biological evaluation. Drug Des Discov 15:199–206
Harris RE (2009) Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate and lung. Inflammopharmacol 17:55–67
Harris RE, Beebe-Donk J, Alshafie GA (2007) Cancer chemoprevention by cyclooxygenase-2 (COX-2) blockade: results of case control studies. Subcell Biochem 42:193–212
Harris RE, Beebe-Donk J, Alshafie GA (2008) Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer 8:237–242
Hegazy GH, Taher A, El-Zaher AA (2005) Synthesis of some floctafenine derivatives of expected anti-inflammatory/analgesic activity. Arch Pharm 338:378–384
Jayasekhar P, Rao SB, Santhakumari G (1997) Synthesis and anti-inflammatory activity of Schiff bases of mesalazine. Indian J Pharma Sci 59:8–12
Jiang H, Cao X, Jiang B (2008) Schiff bases of (+)/(−)-gossybol, it’s preparation and application as antitumor agent. Faming Zhuanli Shenqing Gongkai Shuomingshu. Chem Abstr 150, 20277
Khedekar PB, Baheker RH, Chopade RS, Umathe SN, Rao ARR, Bhusari KP (2003) Synthesis and anti-inflammatory activity of alkyl/arylidene-2-aminobenzothiazoles and 1-benzothiazol-1–2-yl-3-chloro-4-substituted-azetidin-2-ones. Arzneimittel-Forschung 53:640–647
Kumar S, Rajkumar SV (2006) Thalidomide and lenalidomide in the treatment of multiple myeloma. Eur J Cancer 42:1612–1622
Magd–El–Din AA, Atta SMSh, Abd–El-All AS, Gala SA, Abdalah MM (2009) New synthesis of tetrahydrobenzo [4, 5] thieno [2, 3-d] pyrimidine derivatives and schiff bases derived from 2-aminotetrahydro benzothiophenes and hetarylcarbox-aldehydes studies on their antitumor and antimicrobial activities. World J Chem 4:112–117
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P (1991) Feasibility of a high–flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–766
Shalaby AM, El-Eraky W (1997) Studies on O-[2,6-dichlorophenyl–1–amino] phenyl acetic acid I. Synthesis, anti-inflammatory, analgesic and ulcerogenic activities of some new amino acid conjugates. Farmaco 52(2):83–87
Skehan P, Storeng R, Scudiero D, Monks A, McMohan J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Sondhi SM, Singh J, Rani R, Gupta PP, Agarwal SK, Saxena AK (2010a) Synthesis, anti-inflammatory and anticancer activity evaluation of some novel acridine derivatives. Eur J Med Chem 45:555–563
Sondhi SM, Rani R, Singh J, Roy P, Agarwal SK, Saxena AK (2010b) Solvent free synthesis, anti-inflammatory and anticancer activity evaluation of tricyclic and tetracyclic benzimidazole derivatives. Bioorg Med Chem Lett 20:2306–2310
Sondhi SM, Singh J, Agarwal SK, Saxena AK, Roy P (2010) Synthesis of pyrimidine and condensed pyrimidine derivatives and their evaluation for anti-inflammatory and anticancer activities. Med Chem Res. doi:10.1007/s00044-011-9850-7
Sondhi SM, Rani R, Roy P, Agarwal SK, Saxena AK (2010d) Conventional and microwave assisted synthesis of small molecule based biologically active heterocyclic amidine derivatives. Eur J Med Chem 45:902–908
Sondhi SM, Singh J, Roy P, Agarwal SK, Saxena AK (2011) Conventional and microwave assisted synthesis of imidazole and guanidine derivatives and their biological evaluation. Med Chem Res 20:887–897
Tanaka R, Kitagawa H, Sasaki M, Muto S, Itai A, Tokuyama R (2005) Preparation of aromatic or heterocycle imine and amide derivatives as prostaglandin D2 (PGD2) production inhibitors. PCT International Application WO 2005094805. Chem Abstr 143, 387025
Vazzana I, Terranova E, Mattioli F, Sparatore F (2004) Aromatic schiff bases and 2, 3-disubstituted-1,3-thiazolidin-4-one derivatives as anti-inflammatory agents. ARKIVOC 5:364–374
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced Edema in hind paw of rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111:544–547
Wolf GA (ed) (1981) Burger’s medicinal chemistry part III. Wiley, New York, pp 1205–1273
Xiao D, Deguchi A, Gundersen GG, Oehlen B, Arnold L, Weinstein IB (2006) The sulindac derivatives OSI-461, OSIP486823, and OSIP487703 arrest colon cancer cells in mitosis by microtubule depolymerization. Mol Cancer Ther 5:60–67
Acknowledgments
We thank the technical staff of Chemistry Department, IIT Roorkee, for their assistance in spectroscopic studies and elemental analysis and to Mr. Rakesh Kumar (Integral Biosciences Ltd. Noida) for helping to use microwave reactor. One of the authors Ms. Surbhi Arya (JRF-NET) thanks to CSIR, New Delhi, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sondhi, S.M., Arya, S., Rani, R. et al. Synthesis, anti-inflammatory and anticancer activity evaluation of some mono- and bis-Schiff’s bases. Med Chem Res 21, 3620–3628 (2012). https://doi.org/10.1007/s00044-011-9899-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9899-3