Abstract
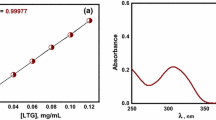
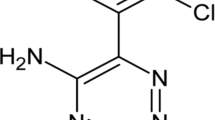
Synthesis of lamotrigine–dextran conjugates is done by oxidation of dextran using sodium periodate (NaIO4), where the aldehyde groups formed were coupled with the amino (–NH2) group of lamotrigine and reduced to secondary imine groups. Characterization of synthesized conjugates was done by using ultraviolet, infrared, nuclear magnetic resonance spectroscopy. Viscometer was used to determine molecular weight, and degree of substitution was estimated by complete hydrolysis of conjugates in borate buffer, which was found to be 8%. Buffer solutions with different pH, i.e., 1.2, 7.4, and 9.0 were used to perform the in vitro hydrolysis study and the amount of conjugates released was estimated by high performance liquid chromatography. The study showed that the rate of hydrolysis increases with increase in pH. The anticonvulsant and hepatotoxicity screening of the synthesized conjugates in different rat models showed that lamotrigine–dextran conjugates proved to be potentially safer than parent lamotrigine formulations.


Similar content being viewed by others
References
Barbosa NR, Mdio AF (2000) Validated high-performance liquid chromatographic method for the determination of lamotrigine in human plasma. J Chromatogr B Biomed Sci Appl 741:289–293
Baudys M, Letourneur D, Liu F, Mix D, Jozefonvicz J, Kim SW (1998) Dextran and 5-aminosalicylic acid (5-ASA) conjugates: synthesis, characterization and enzymic hydrolysis. Bioconj Chem 9:176–183
Betts T, Goodwin G, Withers RM, Yuen AWC (1991a) Human safety of lamotrigine. Epilepsia 32(suppl 2):S17–S22
Betts T, Goodwin G, Withers RM, Yuen AW (1991b) Human safety of lamotrigine. Epilepsia 32:17–21
Fayad M, Choueiri R, Mikati M (2000) Potential hepatotoxicity of lamotrigine. Pediatr Neurol 22:49–52
Makin AJ, Fitt S, Williams R, Duncan JS (1995) Fulminant hepatic failure induced by lamotrigine. Br Med J 311:292
May TW, Rambeck B, Jürgens U (1996) Serum concentrations of lamotrigine in epileptic patients: the influence of dose and comedication. Ther Drug Monit 18(5):523–531
Mehvar R (2000) Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J Control Release 69:1–25
Mehvar R, Dannb RO, Hoga DA (2000) Kinetics of hydrolysis of dextran–methylprednisolone succinate, a macromolecular prodrug of methylprednisolone, in rat blood and liver lysosomes. J Control Release 68:53–61
Meshkibaf MH, Ebrahimi A, Ghodsi R, Ahmadi A (2006) Chronic effects of lamotrigine on liver function in adult male rats. Indian J Clin Biochem 21(1):161–164
Misra GS (1993) Introductory polymer chemistry, 1st edn. New Age International Publishers, p 99
Moeller KE (2008) Acute hepatotoxicity associated with lamotrigine. Am J Psychiatry 165:4
Ouchi T, Ohya Y (1995) Macromolecular prodrugs. Prog Polym Sci 20:211–257
Overstreet K, Costanza C, Behling C, Hassanin T, Masliah E (2002) Fatal progressive hepatic necrosis associated with lamotrigine treatment: a case report and literature review. Dig Dis Sci 47:1921–1925
Sauve G, Bresson S, Prost P (2000) Acute hepatitis after lamotrigine administration. Dig Dis Sci 45:1874–1877
Serwetman LR, Susan AK, Javedan H (2008) Rash and liver dysfunction related to lamotrigine therapy. J Pharm Technol 24:17–21
Walaisiri M, Kirsch LE (2006) The protein-binding and drug release properties of macromolecular conjugates containing daptomycin and dextran. J Pharm 315:30–43
Yuen AWC, Bihari DJ (1992) Multiorgan failure and disseminated intravascular coagulation in severe convulsive seizures. Lancet 340:618
Zimm BH (1948) The scattering of light and redial distribution function of high polymer solution. J Chem Phys 16:1093–1116
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pugazhendhy, S., Shrivastava, P.K., Sinha, S.K. et al. Lamotrigine–dextran conjugates-synthesis, characterization, and biological evaluation. Med Chem Res 20, 595–600 (2011). https://doi.org/10.1007/s00044-010-9355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9355-9