Abstract
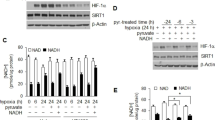
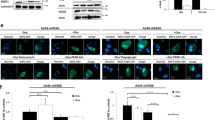
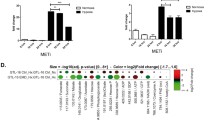
Hypoxia is frequently encountered in the microenvironment of solid tumors. Hypoxia-inducible factors (HIFs), the main effectors of cell response to hypoxia, promote cancer cell survival and progression. HIF-1α, the oxygen-regulated subunit of HIF-1, is often correlated with oncogenesis and represents an attractive therapeutic target. We have previously reported that activation HIF-1α by ERK involves modification of two serine residues and masking of a nuclear export signal (NES), all inside a 43-amino acid domain termed ERK Targeted Domain (ETD). Overexpression of ETD variants including wild-type, phospho-mimetic (SE) or NES-less (IA) mutant forms caused HIF-1 inactivation in two hepatocarcinoma cell lines, while a phospho-deficient (SA) form was ineffective and acted as a sequence-specific negative control. To deliver these ETD forms directly into cancer cells, they were fused to the HIV TAT-sequence and produced as cell-permeable peptides. When the TAT-ETD peptides were added to the culture medium of Huh7 cells, they entered the cells and, with the exception of ETD-SA, accumulated inside the nucleus, caused mislocalization of endogenous HIF-1α to the cytoplasm, significant reduction of HIF-1 activity and inhibition of expression of specific HIF-1, but not HIF-2, gene targets under hypoxia. More importantly, transduced nuclear TAT-ETD peptides restricted migration, impaired colony formation and triggered apoptotic cell death of cancer cells grown under hypoxia, while they produced no effects in normoxic cells. These data demonstrate the importance of ERK-mediated activation of HIF-1 for low oxygen adaptation and the applicability of ETD peptide derivatives as sequence-specific HIF-1 and cancer cell growth inhibitors under hypoxia.







Similar content being viewed by others
References
Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I (2018) The hypoxic tumour microenvironment. Oncogenesis 7(1):10. https://doi.org/10.1038/s41389-017-0011-9
Keith B, Johnson RS, Simon MC (2012) HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev 12(1):9–22
Schofield CJ, Ratcliffe PJ (2005) Signalling hypoxia by HIF hydroxylases. Biochem Biophys Res Commun 338(1):617–626
Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL (1997) Transactivation and inhibitory domains of hypoxia-inducible factor 1 alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem 272(31):19253–19260
Semenza GL (2013) HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123(9):3664–3671. https://doi.org/10.1172/JCI67230
Schito L, Semenza GL (2016) Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer 2(12):758–770. https://doi.org/10.1016/j.trecan.2016.10.016
Poon E, Harris AL, Ashcroft M (2009) Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med 11:e26. https://doi.org/10.1017/S1462399409001173
Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, Hao G, Yousuf Q, Joyce A, Pedrosa I, Geiger H, Zhang H, Chang J, Gardner KH, Bruick RK, Reeves C, Hwang TH, Courtney K, Frenkel E, Sun X, Zojwalla N, Wong T, Rizzi JP, Wallace EM, Josey JA, Xie Y, Xie XJ, Kapur P, McKay RM, Brugarolas J (2016) Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539(7627):112–117. https://doi.org/10.1038/nature19796
Eltzschig HK, Bratton DL, Colgan SP (2014) Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13(11):852–869. https://doi.org/10.1038/nrd4422
Dengler VL, Galbraith MD, Espinosa JM (2014) Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 49(1):1–15. https://doi.org/10.3109/10409238.2013.838205
Kietzmann T, Mennerich D, Dimova EY (2016) Hypoxia-inducible factors (HIFs) and phosphorylation: impact on stability, localization, and transactivity. Front Cell Dev Biol 4:11. https://doi.org/10.3389/fcell.2016.00011
Kalousi A, Mylonis I, Politou AS, Chachami G, Paraskeva E, Simos G (2010) Casein kinase 1 regulates human hypoxia-inducible factor HIF-1. J Cell Sci 123(17):2976–2986. https://doi.org/10.1242/jcs.068122
Mylonis I, Chachami G, Samiotaki M, Panayotou G, Paraskeva E, Kalousi A, Georgatsou E, Bonanou S, Simos G (2006) Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor-1alpha. J Biol Chem 281(44):33095–33106. https://doi.org/10.1074/jbc.M605058200
Mylonis I, Chachami G, Paraskeva E, Simos G (2008) Atypical CRM1-dependent nuclear export signal mediates regulation of hypoxia-inducible factor-1alpha by MAPK. J Biol Chem 283(41):27620–27627. https://doi.org/10.1074/jbc.M803081200
Mylonis I, Sembongi H, Befani C, Liakos P, Siniossoglou S, Simos G (2012) Hypoxia causes triglyceride accumulation by HIF-1-mediated stimulation of lipin 1 expression. J Cell Sci 125(14):3485–3493. https://doi.org/10.1242/jcs.106682
Mylonis I, Kourti M, Samiotaki M, Panayotou G, Simos G (2017) Mortalin-mediated and ERK-controlled targeting of HIF-1alpha to mitochondria confers resistance to apoptosis under hypoxia. J Cell Sci 130(2):466–479. https://doi.org/10.1242/jcs.195339
Mylonis I, Lakka A, Tsakalof A, Simos G (2010) The dietary flavonoid kaempferol effectively inhibits HIF-1 activity and hepatoma cancer cell viability under hypoxic conditions. Biochem Biophys Res Commun 398(1):74–78. https://doi.org/10.1016/j.bbrc.2010.06.038
Rizzuti M, Nizzardo M, Zanetta C, Ramirez A, Corti S (2015) Therapeutic applications of the cell-penetrating HIV-1 Tat peptide. Drug Discov Today 20(1):76–85. https://doi.org/10.1016/j.drudis.2014.09.017
Cabrita LD, Gilis D, Robertson AL, Dehouck Y, Rooman M, Bottomley SP (2007) Enhancing the stability and solubility of TEV protease using in silico design. Protein Sci 16(11):2360–2367. https://doi.org/10.1110/ps.072822507
Hausmann A, Lee J, Pantopoulos K (2011) Redox control of iron regulatory protein 2 stability. FEBS Lett 585(4):687–692. https://doi.org/10.1016/j.febslet.2011.01.036
Andrews NC, Faller DV (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucl Acids Res 19(9):2499
Lyberopoulou A, Venieris E, Mylonis I, Chachami G, Pappas I, Simos G, Bonanou S, Georgatsou E (2007) MgcRacGAP interacts with HIF-1alpha and regulates its transcriptional activity. Cell Physiol Biochem 20(6):995–1006. https://doi.org/10.1159/000110460
Greenspan P, Mayer EP, Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100(3):965–973
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2(2):329–333. https://doi.org/10.1038/nprot.2007.30
Rafehi H, Orlowski C, Georgiadis GT, Ververis K, El-Osta A, Karagiannis TC (2011) Clonogenic assay: adherent cells. J Vis Exp. https://doi.org/10.3791/2573
McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A (2014) Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13(9):1400–1412. https://doi.org/10.4161/cc.28401
Cangul H (2004) Hypoxia upregulates the expression of the NDRG1 gene leading to its overexpression in various human cancers. BMC Genet 5:27. https://doi.org/10.1186/1471-2156-5-27
Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL (2013) Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem 288(15):10819–10829. https://doi.org/10.1074/jbc.M112.442939
Befani C, Liakos P (2018) The role of hypoxia-inducible factor-2 alpha in angiogenesis. J Cell Physiol 233(12):9087–9098. https://doi.org/10.1002/jcp.26805
Loboda A, Jozkowicz A, Dulak J (2010) HIF-1 and HIF-2 transcription factors–similar but not identical. Mol Cell 29(5):435–442. https://doi.org/10.1007/s10059-010-0067-2
Kourti M, Ikonomou G, Giakoumakis NN, Rapsomaniki MA, Landegren U, Siniossoglou S, Lygerou Z, Simos G, Mylonis I (2015) CK1delta restrains lipin-1 induction, lipid droplet formation and cell proliferation under hypoxia by reducing HIF-1alpha/ARNT complex formation. Cell Signal 27(6):1129–1140. https://doi.org/10.1016/j.cellsig.2015.02.017
Gilkes DM, Xiang L, Lee SJ, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL (2014) Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc Natl Acad Sci USA 111(3):E384–E393. https://doi.org/10.1073/pnas.1321510111
Hoffmann C, Mao X, Brown-Clay J, Moreau F, Al Absi A, Wurzer H, Sousa B, Schmitt F, Berchem G, Janji B, Thomas C (2018) Hypoxia promotes breast cancer cell invasion through HIF-1alpha-mediated up-regulation of the invadopodial actin bundling protein CSRP2. Sci Rep 8(1):10191. https://doi.org/10.1038/s41598-018-28637-x
Berg T (2008) Inhibition of transcription factors with small organic molecules. Curr Opin Chem Biol 12(4):464–471. https://doi.org/10.1016/j.cbpa.2008.07.023
Fry DC, Vassilev LT (2005) Targeting protein-protein interactions for cancer therapy. J Mol Med (Berl) 83(12):955–963. https://doi.org/10.1007/s00109-005-0705-x
Johnson RM, Harrison SD, Maclean D (2011) Therapeutic applications of cell-penetrating peptides. Methods Mol Biol 683:535–551. https://doi.org/10.1007/978-1-60761-919-2_38
Kung ALWS, Klco JM, Kaelin WG, Livingston DM (2000) Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med 6(12):1335–1340
Wang X, Qiao Y, Asangani IA, Ateeq B, Poliakov A, Cieslik M, Pitchiaya S, Chakravarthi B, Cao X, Jing X, Wang CX, Apel IJ, Wang R, Tien JC, Juckette KM, Yan W, Jiang H, Wang S, Varambally S, Chinnaiyan AM (2017) Development of peptidomimetic inhibitors of the erg gene fusion product in prostate cancer. Cancer Cell 31(4):532–548.e7. https://doi.org/10.1016/j.ccell.2017.02.017
Hubbi ME, Kshitiz Gilkes DM, Rey S, Wong CC, Luo W, Kim DH, Dang CV, Levchenko A, Semenza GL (2013) A nontranscriptional role for HIF-1 alpha as a direct inhibitor of DNA replication. Sci Signal 6(262):ra10. https://doi.org/10.1126/scisignal.2003417
Triantafyllou EA, Georgatsou E, Mylonis I, Simos G, Paraskeva E (2018) Expression of AGPAT2, an enzyme involved in the glycerophospholipid/triacylglycerol biosynthesis pathway, is directly regulated by HIF-1 and promotes survival and etoposide resistance of cancer cells under hypoxia. Biochim Biophys Acta 1863(9):1142–1152. https://doi.org/10.1016/j.bbalip.2018.06.015
Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li JL, Zhang Q, Wakelam MJO, Karpe F, Schulze A, Harris AL (2014) Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep 9(1):349–365. https://doi.org/10.1016/j.celrep.2014.08.056
Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinnen JV, Smans K (2013) Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res 52(4):585–589. https://doi.org/10.1016/j.plipres.2013.08.005
Rey S, Schito L, Wouters BG, Eliasof S, Kerbel RS (2017) Targeting hypoxia-inducible factors for antiangiogenic cancer therapy. Trends Cancer 3(7):529–541. https://doi.org/10.1016/j.trecan.2017.05.002
Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW (2001) Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther 8(8):638–645. https://doi.org/10.1038/sj.gt.3301388
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT (2002) Regulation of hypoxia-inducible factor 1 alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22(20):7004–7014
Tan C, de Noronha RG, Roecker AJ, Pyrzynska B, Khwaja F, Zhang Z, Zhang H, Teng Q, Nicholson AC, Giannakakou P, Zhou W, Olson JJ, Pereira MM, Nicolaou KC, Van Meir EG (2005) Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res 65(2):605–612
Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM (2002) Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem 277(33):29936–29944. https://doi.org/10.1074/jbc.M204733200
Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL (2009) Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA 106(42):17910–17915. https://doi.org/10.1073/pnas.0909353106
Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G (2005) Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res 65(19):9047–9055. https://doi.org/10.1158/0008-5472.CAN-05-1235
Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, Memmert K, Naegeli HU, Petersen F, Eck MJ, Bair KW, Wood AW, Livingston DM (2004) Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell 6(1):33–43. https://doi.org/10.1016/j.ccr.2004.06.009
Singh V, Nand A, Chen C, Li Z, Li SJ, Wang S, Yang M, Merino A, Zhang L, Zhu J (2014) Echinomycin, a potential binder of FKBP12, shows minor effect on calcineurin activity. J Biomol Screen 19(9):1275–1281. https://doi.org/10.1177/1087057114544742
Yano K, Horinaka M, Yoshida T, Yasuda T, Taniguchi H, Goda AE, Wakada M, Yoshikawa S, Nakamura T, Kawauchi A, Miki T, Sakai T (2011) Chetomin induces degradation of XIAP and enhances TRAIL sensitivity in urogenital cancer cells. Int J Oncol 38(2):365–374. https://doi.org/10.3892/ijo.2010.874
Miranda E, Nordgren IK, Male AL, Lawrence CE, Hoakwie F, Cuda F, Court W, Fox KR, Townsend PA, Packham GK, Eccles SA, Tavassoli A (2013) A cyclic peptide inhibitor of HIF-1 heterodimerization that inhibits hypoxia signaling in cancer cells. J Am Chem Soc 135(28):10418–10425. https://doi.org/10.1021/ja402993u
Rohwer N, Cramer T (2011) Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 14(3):191–201. https://doi.org/10.1016/j.drup.2011.03.001
Zhang M, Qiu Q, Li Z, Sachdeva M, Min H, Cardona DM, DeLaney TF, Han T, Ma Y, Luo L, Ilkayeva OR, Lui K, Nichols AG, Newgard CB, Kastan MB, Rathmell JC, Dewhirst MW, Kirsch DG (2015) HIF-1 alpha regulates the response of primary sarcomas to radiation therapy through a cell autonomous mechanism. Radiat Res 183(6):594–609. https://doi.org/10.1667/RR14016.1
Cho H, Du X, Rizzi JP, Liberzon E, Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA, Wallace EM, Kaelin WG (2016) On-target efficacy of a HIF-2alpha antagonist in preclinical kidney cancer models. Nature 539(7627):107–111. https://doi.org/10.1038/nature19795
Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL (2008) Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA 105(50):19579–19586. https://doi.org/10.1073/pnas.0809763105
Acknowledgements
We are grateful to Prof. K. Pantopoulos (McGill University, Montreal, Quebec, Canada) for offering tTA-HepG2 cells. A.K. is supported by a fellowship from States Scholarship Foundation (ΙΚΥ). This work was partially supported by the “ARISTEIA ΙΙ” Action of the “OPERATIONAL PROGRAM EDUCATION AND LIFELONG LEARNING” (HYPOXYTARGET 3129 to G.S.) co-funded by the European Social Fund (ESF) and National Resources and by a Research Grant in Biomedical Sciences funded by Fondation Santé (5360 to I.M.).
Author information
Authors and Affiliations
Contributions
IM and GS conceived the study and carried out its design. AK and MK performed the experiments. AK, GS and IM analyzed data and wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karagiota, A., Kourti, M., Simos, G. et al. HIF-1α-derived cell-penetrating peptides inhibit ERK-dependent activation of HIF-1 and trigger apoptosis of cancer cells under hypoxia. Cell. Mol. Life Sci. 76, 809–825 (2019). https://doi.org/10.1007/s00018-018-2985-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2985-7