Abstract
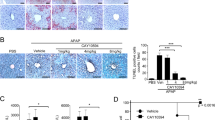
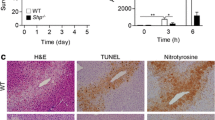
As an analgesic and antipyretic drug, acetaminophen (APAP) is commonly used and known to be safe at therapeutic doses. In many countries, the overuse of APAP provokes acute liver injury and even liver failure. APAP-induced liver injury (AILI) is the most used experimental model of drug-induced liver injury (DILI). Here, we have demonstrated elevated levels of growth arrest and DNA damage-inducible 45α (GADD45α) in the livers of patients with DILI/AILI, in APAP-injured mouse livers and in APAP-treated hepatocytes. GADD45α exhibited a protective effect against APAP-induced liver injury and alleviated the accumulation of small lipid droplets in vitro and in vivo. We found that GADD45α promoted the activation of AMP-activated protein kinase α and induced fatty acid beta-oxidation, tricarboxylic acid cycle (TCA) and glycogenolysis-related gene expression after APAP exposure. Liquid chromatography–mass spectrometry (LC–MS) analysis showed that GADD45α increased the levels of TCA cycle metabolites. Co-immunoprecipitation analysis showed that Ppp2cb, a catalytic subunit of protein phosphatase 2A, could interact directly with GADD45α. Our results indicate that hepatocyte GADD45α might represent a therapeutic target to prevent and rescue liver injury caused by APAP.
Graphical abstract









Similar content being viewed by others
References
Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S (2013) Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 144(7):1419–1425. https://doi.org/10.1053/j.gastro.2013.02.006 (1425 e1411–1413; quiz e1419–1420)
Lin CC, Tsai P, Sun HY, Hsu MC, Lee JC, Wu IC, Tsao CW, Chang TT, Young KC (2014) Apolipoprotein J, a glucose-upregulated molecular chaperone, stabilizes core and NS5A to promote infectious hepatitis C virus virion production. J Hepatol 61(5):984–993. https://doi.org/10.1016/j.jhep.2014.06.026
Bernal W, Auzinger G, Dhawan A, Wendon J (2010) Acute liver failure. Lancet 376(9736):190–201. https://doi.org/10.1016/S0140-6736(10)60274-7
Jaeschke H, McGill MR, Williams CD, Ramachandran A (2011) Current issues with acetaminophen hepatotoxicity—a clinically relevant model to test the efficacy of natural products. Life Sci 88(17–18):737–745. https://doi.org/10.1016/j.lfs.2011.01.025
Masubuchi Y, Suda C, Horie T (2005) Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol 42(1):110–116. https://doi.org/10.1016/j.jhep.2004.09.015
Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, Wang Y, Lam KS, Xu A (2014) Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1alpha-mediated antioxidant capacity in mice. Hepatology 60(3):977–989. https://doi.org/10.1002/hep.27060
Yuan L, Kaplowitz N (2013) Mechanisms of drug-induced liver injury. Clin Liver Dis 17(4):507–518. https://doi.org/10.1016/j.cld.2013.07.002 (vii)
Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B (2011) Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol 54(4):773–794. https://doi.org/10.1016/j.jhep.2010.11.006
Zhang X, Ouyang J, Thung SN (2013) Histopathologic manifestations of drug-induced hepatotoxicity. Clin Liver Dis 17(4):547–564. https://doi.org/10.1016/j.cld.2013.07.004 (vii–viii)
Chen C, Krausz KW, Shah YM, Idle JR, Gonzalez FJ (2009) Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol 22(4):699–707. https://doi.org/10.1021/tx800464q
Salvador JM, Brown-Clay JD, Fornace AJ Jr (2013) Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv Exp Med Biol 793:1–19. https://doi.org/10.1007/978-1-4614-8289-5_1
Schafer A (2013) Gadd45 proteins: key players of repair-mediated DNA demethylation. Adv Exp Med Biol 793:35–50. https://doi.org/10.1007/978-1-4614-8289-5_3
Zhang L, Yang Z, Liu Y (2014) GADD45 proteins: roles in cellular senescence and tumor development. Exp Biol Med 239(7):773–778. https://doi.org/10.1177/1535370214531879
Tian J, Locker J (2013) Gadd45 in the liver: signal transduction and transcriptional mechanisms. Adv Exp Med Biol 793:69–80. https://doi.org/10.1007/978-1-4614-8289-5_5
Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ Jr (1992) A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71(4):587–597
Oh-Hashi K, Maruyama W, Isobe K (2001) Peroxynitrite induces GADD34, 45, and 153 VIA p38 MAPK in human neuroblastoma SH-SY5Y cells. Free Radic Biol Med 30(2):213–221
Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD, Haber DA (1999) Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell 97(5):575–586
Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massague J (2006) A FoxO–Smad synexpression group in human keratinocytes. Proc Natl Acad Sci USA 103(34):12747–12752. https://doi.org/10.1073/pnas.0605333103
Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, Kastan MB, O’Connor PM, Fornace AJ Jr (1994) Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science 266(5189):1376–1380
Vairapandi M, Azam N, Balliet AG, Hoffman B, Liebermann DA (2000) Characterization of MyD118, Gadd45, and proliferating cell nuclear antigen (PCNA) interacting domains. PCNA impedes MyD118 AND Gadd45-mediated negative growth control. J Biol Chem 275(22):16810–16819
Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, Niehrs C (2007) Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 445(7128):671–675. https://doi.org/10.1038/nature05515
Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, Hollander MC, O’Connor PM, Fornace AJ Jr, Harris CC (1999) GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA 96(7):3706–3711
Zhan Q, Antinore MJ, Wang XW, Carrier F, Smith ML, Harris CC, Fornace AJ Jr (1999) Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene 18(18):2892–2900. https://doi.org/10.1038/sj.onc.1202667
Maeda T, Hanna AN, Sim AB, Chua PP, Chong MT, Tron VA (2002) GADD45 regulates G2/M arrest, DNA repair, and cell death in keratinocytes following ultraviolet exposure. J Investig Dermatol 119(1):22–26. https://doi.org/10.1046/j.1523-1747.2002.01781.x
Zhu N, Shao Y, Xu L, Yu L, Sun L (2009) Gadd45-alpha and Gadd45-gamma utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol Biol Rep 36(8):2075–2085. https://doi.org/10.1007/s11033-008-9419-9
Tong T, Ji J, Jin S, Li X, Fan W, Song Y, Wang M, Liu Z, Wu M, Zhan Q (2005) Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol Cell Biol 25(11):4488–4500. https://doi.org/10.1128/MCB.25.11.4488-4500.2005
Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L, Fornace AJ Jr (2002) Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Can Res 62(24):7305–7315
Pessayre D, Fromenty B, Berson A, Robin MA, Letteron P, Moreau R, Mansouri A (2012) Central role of mitochondria in drug-induced liver injury. Drug Metab Rev 44(1):34–87. https://doi.org/10.3109/03602532.2011.604086
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89(3):1025–1078. https://doi.org/10.1152/physrev.00011.2008
Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, Viollet B, Guigas B (2011) Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 54(12):3101–3110. https://doi.org/10.1007/s00125-011-2311-5
Dzamko N, van Denderen BJ, Hevener AL, Jorgensen SB, Honeyman J, Galic S, Chen ZP, Watt MJ, Campbell DJ, Steinberg GR, Kemp BE (2010) AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem 285(1):115–122. https://doi.org/10.1074/jbc.M109.056762
Hardie DG (2015) AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol 33:1–7. https://doi.org/10.1016/j.ceb.2014.09.004
Hardie DG (2014) AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr 34:31–55. https://doi.org/10.1146/annurev-nutr-071812-161148
Clarke PR, Hardie DG (1990) Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J 9(8):2439–2446
Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, Giordanetto F, Martin SR, Carling D, Gamblin SJ (2013) Structural basis of AMPK regulation by small molecule activators. Nat Commun 4:3017. https://doi.org/10.1038/ncomms4017
Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, Hardie DG (2012) The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336(6083):918–922. https://doi.org/10.1126/science.1215327
Hou J, Zhou Y, Zheng Y, Fan J, Zhou W, Ng IO, Sun H, Qin L, Qiu S, Lee JM, Lo CM, Man K, Yang Y, Yang Y, Yang Y, Zhang Q, Zhu X, Li N, Wang Z, Ding G, Zhuang SM, Zheng L, Luo X, Xie Y, Liang A, Wang Z, Zhang M, Xia Q, Liang T, Yu Y, Cao X (2014) Hepatic RIG-I predicts survival and interferon-alpha therapeutic response in hepatocellular carcinoma. Cancer Cell 25(1):49–63. https://doi.org/10.1016/j.ccr.2013.11.011
Han Q, Zhang C, Zhang J, Tian Z (2011) Reversal of hepatitis B virus-induced immune tolerance by an immunostimulatory 3p-HBx-siRNAs in a retinoic acid inducible gene I-dependent manner. Hepatology 54(4):1179–1189. https://doi.org/10.1002/hep.24505
Wang Z, Yang X, Chen L, Zhi X, Lu H, Ning Y, Yeong J, Chen S, Yin L, Wang X, Li X (2017) Upregulation of hydroxysteroid sulfotransferase 2B1b promotes hepatic oval cell proliferation by modulating oxysterol-induced LXR activation in a mouse model of liver injury. Arch Toxicol 91(1):271–287. https://doi.org/10.1007/s00204-016-1693-z
Li X, Hylemon P, Pandak WM, Ren S (2006) Enzyme activity assay for cholesterol 27-hydroxylase in mitochondria. J Lipid Res 47(7):1507–1512. https://doi.org/10.1194/jlr.M600117-JLR200
McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H (2012) The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Investig 122(4):1574–1583. https://doi.org/10.1172/JCI59755
Cohen PT, Brewis ND, Hughes V, Mann DJ (1990) Protein serine/threonine phosphatases; an expanding family. FEBS Lett 268(2):355–359
de Achaval S, Suarez-Almazor M (2011) Acetaminophen overdose: a little recognized public health threat. Pharmacoepidemiol Drug Saf 20(8):827–829. https://doi.org/10.1002/pds.2162
Jaeschke H, Williams CD, Ramachandran A, Bajt ML (2012) Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int Off J Int Assoc Study Liver 32(1):8–20. https://doi.org/10.1111/j.1478-3231.2011.02501.x
Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U (2014) Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol 184(11):3013–3025. https://doi.org/10.1016/j.ajpath.2014.07.019
Jin S, Antinore MJ, Lung FD, Dong X, Zhao H, Fan F, Colchagie AB, Blanck P, Roller PP, Fornace AJ Jr, Zhan Q (2000) The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem 275(22):16602–16608. https://doi.org/10.1074/jbc.M000284200
Su LY, Xin HY, Liu YL, Zhang JL, Xin HW, Su XL (2014) Anticancer bioactive peptide (ACBP) inhibits gastric cancer cells by upregulating growth arrest and DNA damage-inducible gene 45A (GADD45A). Tumour Biol J Int Soc Oncodev Biol Med 35(10):10051–10056. https://doi.org/10.1007/s13277-014-2272-7
Maekawa T, Sano Y, Shinagawa T, Rahman Z, Sakuma T, Nomura S, Licht JD, Ishii S (2008) ATF-2 controls transcription of Maspin and GADD45 alpha genes independently from p53 to suppress mammary tumors. Oncogene 27(8):1045–1054. https://doi.org/10.1038/sj.onc.1210727
Hong L, Sun QF, Xu TY, Wu YH, Zhang H, Fu RQ, Cai FJ, Zhou QQ, Zhou K, Du QW, Zhang D, Xu S, Ding JG (2016) New role and molecular mechanism of Gadd45a in hepatic fi brosis. World J Gastroenterol 22(9):2779–2788. https://doi.org/10.3748/wjg.v22.i9.2779
Tanaka N, Takahashi S, Hu X, Lu Y, Fujimori N, Golla S, Fang ZZ, Aoyama T, Krausz KW, Gonzalez FJ (2017) Growth arrest and DNA damage-inducible 45alpha protects against nonalcoholic steatohepatitis induced by methionine- and choline-deficient diet. Biochem Biophys Acta 1863 12:3170–3182. https://doi.org/10.1016/j.bbadis.2017.08.017
Pang C, Shi L, Sheng Y, Zheng Z, Wei H, Wang Z, Ji L (2016) Caffeic acid attenuated acetaminophen-induced hepatotoxicity by inhibiting ERK1/2-mediated early growth response-1 transcriptional activation. Chem Biol Interact 260:186–195. https://doi.org/10.1016/j.cbi.2016.10.009
Towler MC, Hardie DG (2007) AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100(3):328–341. https://doi.org/10.1161/01.RES.0000256090.42690.05
Yang YM, Han CY, Kim YJ, Kim SG (2010) AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol 16(30):3731–3742
Nakada D, Saunders TL, Morrison SJ (2010) Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468(7324):653–658. https://doi.org/10.1038/nature09571
Choi SH, Kim YW, Kim SG (2010) AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol 79(9):1352–1362. https://doi.org/10.1016/j.bcp.2009.12.011
Hoyer-Hansen M, Jaattela M (2007) AMP-activated protein kinase: a universal regulator of autophagy? Autophagy 3(4):381–383
Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N (2014) Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and -independent signaling pathways. Hepatology 59(4):1543–1554. https://doi.org/10.1002/hep.26625
Hwang JH, Kim YH, Noh JR, Choi DH, Kim KS, Lee CH (2015) Enhanced production of adenosine triphosphate by pharmacological activation of adenosine monophosphate-activated Protein kinase ameliorates acetaminophen-induced liver injury. Mol Cells 38(10):843–850. https://doi.org/10.14348/molcells.2015.0072
Hardie DG (2008) AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes 32(Suppl 4):S7–12. https://doi.org/10.1038/ijo.2008.116
Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1(1):15–25. https://doi.org/10.1016/j.cmet.2004.12.003
Qiang X, Xu L, Zhang M, Zhang P, Wang Y, Wang Y, Zhao Z, Chen H, Liu X, Zhang Y (2016) Demethyleneberberine attenuates non-alcoholic fatty liver disease with activation of AMPK and inhibition of oxidative stress. Biochem Biophys Res Commun 472(4):603–609. https://doi.org/10.1016/j.bbrc.2016.03.019
Zhang S, Chen G, Li N, Dai M, Chen C, Wang P, Tang H, Hoopes SL, Zeldin DC, Wang DW, Xu X (2015) CYP2J2 overexpression ameliorates hyperlipidemia via increased fatty acid oxidation mediated by the AMPK pathway. Obesity 23(7):1401–1413. https://doi.org/10.1002/oby.21115
Tamura S, Tsuiki S (1980) Purification and subunit structure of rat-liver phosphoprotein phosphatase, whose molecular weight is 260000 by gel filtration (phosphatase IB). Eur J Biochem 111(1):217–224
Tamura S, Kikuchi H, Kikuchi K, Hiraga A, Tsuiki S (1980) Purification and subunit structure of a high-molecular-weight phosphoprotein phosphatase (phosphatase II) from rat liver. Eur J Biochem 104(2):347–355
Moore F, Weekes J, Hardie DG (1991) Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur J Biochem 199(3):691–697
Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D (2007) Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403(1):139–148. https://doi.org/10.1042/BJ20061520
Joseph BK, Liu HY, Francisco J, Pandya D, Donigan M, Gallo-Ebert C, Giordano C, Bata A, Nickels JT Jr (2015) Inhibition of AMP Kinase by the Protein Phosphatase 2A Heterotrimer, PP2APpp2r2d. J Biol Chem 290(17):10588–10598. https://doi.org/10.1074/jbc.M114.626259
Acknowledgements
This work was supported by the Major Project of National Twelfth Five Plan (2012ZX09303-001), the Major Project of National Thirteenth Five Plan (2017ZX09304016), the National Natural Science Foundation of China (NSFC 81670524, NSFC 31771308), the Shanghai Municipal Natural Science Foundation (17ZR1401800) and the Clinical Research Center at Shanghai Jiao Tong University School of Medicine. The funders had no role in the study design, data collection and analysis, decision to publish or manuscript preparation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, C., Ming, Y., Wang, Z. et al. GADD45α alleviates acetaminophen-induced hepatotoxicity by promoting AMPK activation. Cell. Mol. Life Sci. 76, 129–145 (2019). https://doi.org/10.1007/s00018-018-2912-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2912-y