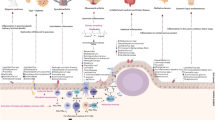
Abstract
Auto-antibodies to the ubiquitous enzyme type-2 transglutaminase (TG2) are a specific hallmark of celiac disease (CD), a widely diffused, multi-factorial disease, affecting genetically predisposed subjects. In CD an inflammatory response, at the intestinal level, is triggered by diet consumption of gluten-containing cereals. Intestinal mucosa displays various degrees of atrophy and hyperplasia, with consequent global intestinal dysfunction and other relevant extra-intestinal symptoms. Through deamidation of specific glutamines of gluten-derived gliadin peptides, TG2 strongly enhances gliadin immunogenicity. In addition, TG2 cross-linking activity may generate complexes between TG2 itself and gliadin peptides, and these complexes seem to cause the auto-immune response by means of an apten-carrier-like mechanism of antigen presentation. Anti-TG2 antibodies can be early detected in the intestinal mucosa of celiac patients and are also abundantly present into the serum, thus potentially reaching other organs and tissues by blood circulation. Recently, the possible pathogenetic role of auto-antibodies to TG2 in CD has been investigated. Here, we report an overview about the genesis of these antibodies, their specificity, their modulating ability toward TG2 enzymatic or non-enzymatic activities and their biological effects exerted by interacting with extracellular TG2 or with cell-surface TG2. We also discuss the auto-immune response occurring in CD against other TG members (i.e. type 3 and type 6) and analyze the occurrence of anti-TG2 antibodies in other auto-immune CD-related diseases. Data now available let us to suppose that, even if antibodies to TG2 do not represent the triggering molecules in CD, they could be important players in disease progression and manifestations.



Similar content being viewed by others
Abbreviations
- TG2:
-
Type-2 transglutaminase
- CD:
-
Celiac disease
- TG:
-
Transglutaminase
- HLA:
-
Human leukocyte antigens
- DH:
-
Dermatitis herpetiformis
- TG3:
-
Type 3 transglutaminase
- TG6:
-
Type 6 transglutaminase
- T1D:
-
Type 1 diabetes
References
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4:140–156. https://doi.org/10.1038/nrm1014
Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D (1997) Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 3(7):797–801. https://doi.org/10.1038/nm0797-797
Grenard P, Bates MK, Aeschlimann D (2001) Evolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z. J Biol Chem 276(35):33066–33078. https://doi.org/10.1074/jbc.M102553200
Lai TS, Lin CJ, Greenberg CS (2017) Role of tissue transglutaminase-2 (TG2)-mediated aminylation in biological processes. Amino Acids 49(3):501–551. https://doi.org/10.1007/s00726-016-2270-8
Stamnaes J, Fleckenstein B, Sollid LM (2008) The propensity for deamidation and transamidation of peptides by transglutaminase 2 is dependent on substrate affinity and reaction conditions. Biochim Biophys Acta 1784(11):1804–1811. https://doi.org/10.1016/j.bbapap.2008.08.011
Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (2011) Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev 91(3):931–972. https://doi.org/10.1152/physrev.00016.2010
Dean MD (2013) Genetic disruption of the copulatory plug in mice leads to severely reduced fertility. PLoS Genet 9(1):e1003185. https://doi.org/10.1371/journal.pgen.1003185
Jiang WG, Ablin RJ (2011) Prostate transglutaminase: a unique transglutaminase and its role in prostate cancer. Biomark Med 5(3):285–291. https://doi.org/10.2217/bmm.11.36
Candi E, Schmidt R, Melino G (2005) The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6(4):328–340. https://doi.org/10.1038/nrm1619
Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GV, Mehta K (2014) Transglutaminase regulation of cell function. Physiol Rev 94(2):383–417. https://doi.org/10.1152/physrev.00019.2013
Ientile R, Caccamo D, Griffin M (2007) Tissue transglutaminase and the stress response. Amino Acids 33(2):385–394. https://doi.org/10.1007/s00726-007-0517-0
Nurminskaya MV, Belkin AM (2012) Cellular functions of tissue transglutaminase. Int Rev Cell Mol Biol 294:1–97. https://doi.org/10.1016/B978-0-12-394305-7.00001-X
Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM (1994) Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science 264(5165):1593–1596
Iismaa SE, Mearns BM, Lorand L, Graham RM (2009) Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev 89(3):991–1023. https://doi.org/10.1152/physrev.00044.2008
Liu S, Cerione RA, Clardy J (2002) Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc Natl Acad Sci USA 99(5):2743–2747. https://doi.org/10.1073/pnas.042454899
Pinkas DM, Strop P, Brunger AT, Khosla C (2007) Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol 5(12):e327. https://doi.org/10.1371/journal.pbio.0050327
Kuo TF, Tatsukawa H, Kojima S (2011) New insights into the functions and localization of nuclear transglutaminase 2. FEBS J 278(24):4756–4767. https://doi.org/10.1111/j.1742-4658.2011.08409.x
Kanchan K, Fuxreiter M, Fésüs L (2015) Physiological, pathological, and structural implications of non-enzymatic protein-protein interactions of the multifunctional human transglutaminase 2. Cell Mol Life Sci 72(16):3009–3035. https://doi.org/10.1007/s00018-015-1909-z
RodolfoC Mormone E, Matarrese P, Ciccosanti F, Farrace MG, Garofano E, Piredda L, Fimia GM, Malorni W, Piacentini M (2004) Tissue transglutaminase is a multifunctional BH3-only protein. J Biol Chem 279:54783–54792. https://doi.org/10.1074/jbc.M410938200
Hasegawa G, Suwa M, Ichikawa Y, Ohtsuka T, Kumagai S, Kikuchi M, Sato Y, Saito Y (2003) A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J 373:793–803. https://doi.org/10.1042/BJ20021084
Mastroberardino PG, Farrace MG, Viti I, Pavone F, Fimia GM, Melino G, Rodolfo C, Piacentini M (2006) “Tissue” transglutaminase contributes to the formation of disulphide bridges in proteins of mitochondrial respiratory complexes. Biochim Biophys Acta 1757:1357–1365. https://doi.org/10.1016/j.bbabio.2006.07.007
Lai TS, Lin CJ, Wu YT, Wu CJ (2017) Tissue transglutaminase (TG2) and mitochondrial function and dysfunction. Front Biosci (Landmark Ed) 1(22):1114–11372017
Zemskov EA, Mikhailenko I, Hsia RC, Zaritskaya L, Belkin AM (2011) Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One 6(4):e19414. https://doi.org/10.1371/journal.pone.0019414
Akimov SS, Krylov D, Fleischman LF, Belkin AM (2000) Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148(4):825–838
Zemskov EA, Mikhailenko I, Smith EP, Belkin AM (2012) Tissue transglutaminase promotes PDGF/PDGFR-mediated signaling and responses in vascular smooth muscle cells. J Cell Physiol 227(5):2089–2096. https://doi.org/10.1002/jcp.22938
Dardik R, Inbal A (2006) Complex formation between tissue transglutaminase II (tTG) and vascular endothelial growth factor receptor 2 (VEGFR-2): proposed mechanism for modulation of endothelial cell response to VEGF. Exp Cell Res 312(16):2973–2982. https://doi.org/10.1016/j.yexcr.2006.05.019
Zemskov EA, Mikhailenko I, Strickland DK, Belkin AM (2007) Cell-surface transglutaminase undergoes internalization and lysosomal degradation: an essential role for LRP1. J Cell Sci 120(Pt 18):3188–3199. https://doi.org/10.1242/jcs.010397
Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M (2008) Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett 582(10):1552–1557. https://doi.org/10.1016/j.febslet.2008.03.053
Scarpellini A, Germack R, Lortat-Jacob H, Muramatsu T, Billett E, Johnson T, Verderio EA (2009) Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J Biol Chem 284(27):18411–18423. https://doi.org/10.1074/jbc.M109.012948
Belkin AM (2011) Extracellular TG2: emerging functions and regulation. FEBS J 278(24):4704–4716. https://doi.org/10.1111/j.1742-4658.2011.08346.x
Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C (2008) Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One 3(3):e1861. https://doi.org/10.1371/journal.pone.000186132
Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM (2010) Redox regulation of transglutaminase 2 activity. J Biol Chem 285:25402–25409. https://doi.org/10.1074/jbc.M109.097162
Fraij BM, Gonzales RA (1996) A third human tissue transglutaminase homologue as a result of alternative gene transcripts. Biochim Biophys Acta 1306:63–74
Monsonego A, Shani Y, Friedmann I, Paas Y, Eizenberg O, Schwartz M (1997) Expression of GTPdependent and GTP-independent tissue-type transglutaminase in cytokine-treated rat brain astrocytes. J Biol Chem 272:3724–3732
Antonyak MA, Jansen JM, Miller AM, Ly TK, Endo M, Cerione RA (1996) Two isoforms of tissue transglutaminase mediate opposing cellular fates. Proc Natl Acad Sci USA 103:18609–18614
Lai TS, Liu Y, Li W, Greenberg CS (2007) Identification of two GTP-independent alternatively spliced forms of tissue transglutaminase in human leukocytes, vascular smooth muscle, and endothelial cells. FASEB J 21:4131–4143
Mishra S, Murphy LJ (2004) Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem 279:23863–23868. https://doi.org/10.1074/jbc.m311919200
Mishra S, Saleh A, Espino PS, Davie JR, Murphy LJ (2006) Phosphorylation of histones by tissue transglutaminase. J Biol Chem 281:5532–5538. https://doi.org/10.1074/jbc.m506864200
Mishra S, Melino G, Murphy LJ (2007) Transglutaminase 2 kinase activity facilitates protein kinaseA-induced phosphorylation of retinoblastoma protein. J Biol Chem 282:18108–18115. https://doi.org/10.1074/jbc.m607413200
Mishra S, Murphy LJ (2006) The p53 oncoprotein is a substrate for tissue transglutaminase kinase activity. Biochem Biophys Res Commun 339:726–730. https://doi.org/10.1016/j.bbrc.2005.11.071
Király R, Thangaraju K, Nagy Z, Collighan R, Nemes Z, Griffin M, Fésüs L (2016) Isopeptidase activity of human transglutaminase 2: disconnection from transamidation and characterization by kinetic parameters. Amino Acids 48(1):31–40. https://doi.org/10.1007/s00726-015-2063-5
Esposito C, Caputo I (2005) Mammalian transglutaminases. Identification of substrates as a key to physiological function and physiopathological relevance. FEBS J 272(3):615–631. https://doi.org/10.1111/j.1742-4658.2004.04476.x
Kumar S, Mehta K (2013) Tissue transglutaminase, inflammation, and cancer: how intimate is the relationship? Amino Acids 44(1):81–88. https://doi.org/10.1007/s00726-011-1139-0
Ruan Q, Johnson GV (2007) Transglutaminase 2 in neurodegenerative disorders. Front Biosci 12:891–904. https://doi.org/10.2741/2111
Sollid LM (2002) Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2(9):647–655. https://doi.org/10.1038/nri885
Leffler DA, Green PH, Fasano A (2015) Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol 12(10):561–571. https://doi.org/10.1038/nrgastro.2015.131
Wieser H (2007) Chemistry of gluten proteins. Food Microbiol 24(2):115–119. https://doi.org/10.1016/j.fm.2006.07.004
Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C (2002) Structural basis for gluten intolerance in celiac sprue. Science 297(5590):2275–2279. https://doi.org/10.1126/science.1074129
Setty M, Discepolo V, Abadie V, Kamhawi S, Mayassi T, Kent A, Ciszewski C, Maglio M, Kistner E, Bhagat G, Semrad C, Kupfer SS, Green PH, Guandalini S, Troncone R, Murray JA, Turner JR, Jabri B (2015) Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intraepithelial killer cells and active celiac disease. Gastroenterology 149(3):681–691. https://doi.org/10.1053/j.gastro.2015.05.013
Schuppan D, Junker Y, Barisani D (2009) Celiac disease: from pathogenesis to novel therapies. Gastroenterology 137(6):1912–1933. https://doi.org/10.1053/j.gastro.2009.09.008
Kaukinen K, Partanen J, Mäki M, Collin P (2002) HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol 97(3):695–699. https://doi.org/10.1111/j.1572-0241.2002.05471.x
Romanos J, Rosén A, Kumar V, Trynka G, Franke L, Szperl A, Gutierrez-Achury J, van Diemen CC, Kanninga R, Jankipersadsing SA, Steck A, Eisenbarth G, van Heel DA, Cukrowska B, Bruno V, Mazzilli MC, Núñez C, Bilbao JR, Mearin ML, Barisani D, Rewers M, Norris JM, Ivarsson A, Boezen HM, Liu E, Wijmenga C, Prevent CD Group (2014) Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut 63(3):415–422. https://doi.org/10.1136/gutjnl-2012-304110
Giersiepen K, Lelgemann M, Stuhldreher N, Ronfani L, Husby S, Koletzko S, Korponay-Szabó IR (2012) ESPGHAN Working Group on Coeliac Disease Diagnosis. Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr 54(2):229–241. https://doi.org/10.1097/MPG.0b013e318216f2e5
Werkstetter KJ, Korponay-Szabó IR, Popp A, Villanacci V, Salemme M, Heilig G, Lillevang ST, Mearin ML, Ribes-Koninckx C, Thomas A, Troncone R, Filipiak B, Mäki M, Gyimesi J, Najafi M, Dolinšek J, Dydensborg Sander S, Auricchio R, Papadopoulou A, Vécsei A, Szitanyi P, Donat E, Nenna R, Alliet P, Penagini F, Garnier-Lengliné H, Castillejo G, Kurppa K, Shamir R, Hauer AC, Smets F, Corujeira S, van Winckel M, Buderus S, Chong S, Husby S, Koletzko S, ProCeDE study group (2017) Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology 153(4):924–935. https://doi.org/10.1053/j.gastro.2017.06.002
Vader LW, de Ru A, van der Wal Y, Kooy YM, Benckhuijsen W, Mearin ML, Drijfhout JW, van Veelen P, Koning F (2002) Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med 195(5):643–92002
Sollid LM, Jabri B (2011) Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of auto-immune disorders. Curr Opin Immunol 23(6):732–738. https://doi.org/10.1016/j.coi.2011.08.006
Sollid LM, Qiao SW, Anderson RP, Gianfrani C, Koning F (2012) Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 64:455–460. https://doi.org/10.1007/s00251-012-0599-z
Fleckenstein B, Molberg Ø, Qiao SW, Schmid DG, von der Mülbe F, Elgstøen K, Jung G, Sollid LM (2002) Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J Biol Chem 277(37):34109–34116. https://doi.org/10.1074/jbc.m204521200
Esposito C, Paparo F, Caputo I, Porta R, Salvati VM, Mazzarella G, Auricchio S, Troncone R (2003) Expression and enzymatic activity of small intestinal tissue transglutaminase in celiac disease. Am J Gastroenterol 98(8):1813–1820. https://doi.org/10.1111/j.1572-0241.2003.07582.x
Biagi F, Campanella J, Laforenza U, Gastaldi G, Tritto S, Grazioli M, Villanacci V, Corazza GR (2006) Transglutaminase 2 in the enterocytes is coeliac specific and gluten dependent. Dig Liver Dis 38(9):652–658. https://doi.org/10.1016/j.dld.2006.05.021
Villanacci V, Not T, Sblattero D, Gaiotto T, Chirdo F, Galletti A, Bassotti G (2009) Mucosal tissue transglutaminase expression in celiac disease. J Cell Mol Med 13(2):334–340. https://doi.org/10.1111/j.1582-4934.2008.00325.x
Dahle C, Hagman A, Ignatova S, Ström M (2010) Antibodies against deamidated gliadin peptides identify adult coeliac disease patients negative for antibodies against endomysium and tissue transglutaminase. Aliment Pharmacol Ther 32(2):254–260. https://doi.org/10.1111/j.1365-2036.2010.04337.x
Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A (2001) Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol 166:4170–4176
Korponay-Szabó IR, Halttunen T, Szalai Z, Laurila K, Király R, Kovács JB, Fésüs L, Mäki M (2004) In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac auto-antibodies. Gut 53(5):641–648. https://doi.org/10.1136/gut.2003.024836
Kaukinen K, Peräaho M, Collin P, Partanen J, Woolley N et al (2005) Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scand J Gastroenterol 40:564–572. https://doi.org/10.1080/00365520510023422
Taavela J, Kurppa K, Collin P, Lähdeaho ML, Salmi T, Saavalainen P, Haimila K, Huhtala H, Laurila K, Sievänen H, Mäki M, Kaukinen K (2013) Degree of damage to the small bowel and serum antibody titers correlate with clinical presentation of patients with celiac disease. Clin Gastroenterol Hepatol 11(2):166–171. https://doi.org/10.1016/j.cgh.2012.09.030
Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N (2002) Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med 195(6):747–757. https://doi.org/10.1084/jem.20011299
Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D (2008) Auto-antibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol 64:332–343. https://doi.org/10.1002/ana.21450
Sollid LM, Molberg Ø, McAdam S, Lundin KEA (1997) Auto-antibodies in celiac disease: tissue transglutaminase–guilt by association? Gut 41:851–852
Fleckenstein B, Qiao SW, Larsen MR, Jung G, Roepstorff P, Sollid LM (2004) Molecular characterization of covalent complexes between tissue transglutaminase and gliadin peptides. J Biol Chem 279:17607–17616. https://doi.org/10.1074/jbc.M310198200
Stamnaes J, Iversen R, du Pré MF, Chen X, Sollid LM (2015) Enhanced B-cell receptor recognition of the autoantigen transglutaminase 2 by efficient catalytic self-multimerization. PLoS One 10(8):e0134922. https://doi.org/10.1371/journal.pone.0134922
Sollid LM (2017) The roles of MHC class II genes and post-translational modification in celiac disease. Immunogenetics 69(8–9):605–616. https://doi.org/10.1007/s00251-017-0985-7
Shaoul R, Lerner A (2007) Associated auto-antibodies in celiac disease. Autoimmun Rev 6(8):559–565. https://doi.org/10.1016/j.autrev.2007.02.006
Alaedini A, Green PH (2008) Auto-antibodies in celiac disease. Autoimmunity 41(1):19–26. https://doi.org/10.1080/08916930701619219
Stamnaes J, Sollid LM (2015) Celiac disease: autoimmunity in response to food antigen. Semin Immunol 27(5):343–352. https://doi.org/10.1016/j.smim.2015.11.001
Iversen R, du Pré MF, Di Niro R, Sollid LM (2015) Igs as substrates for transglutaminase 2: implications for autoantibody production in celiac disease. J Immunol 195:5159–5168. https://doi.org/10.4049/jimmunol.1501363
Zanoni G, Navone R, Lunardi C, Tridente G, Bason C, Sivori S, Beri R, Dolcino M, Valletta E, Corrocher R, Puccetti A (2006) In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med 3(9):e358. https://doi.org/10.1371/journal.pmed.0030358
Dolcino M, Zanoni G, Bason C, Tinazzi E, Boccola E, Valletta E, Contreas G, Lunardi C, Puccetti A (2013) A subset of anti-rotavirus antibodies directed against the viral protein VP7 predicts the onset of celiac disease and induces typical features of the disease in the intestinal epithelial cell line T84. Immunol Res 56(2–3):465–476. https://doi.org/10.1007/s12026-013-8420-0
Korponay-Szabó IR, Vecsei A, Kiraly R, Dahlbom I, Chirdo F, Nemes E, Fésüs L, Maki M (2008) Deamidated gliadin peptides form epitopes that transglutaminase antibodies recognize. J Pediatr Gastroenterol Nutr 46:253–261. https://doi.org/10.1097/mpg.0b013e31815ee555
Seissler J, Wohlrab U, Wuensche C, Scherbaum WA, Boehm BO (2001) Auto-antibodies from patients with coeliac disease recognize distinct functional domains of the autoantigen tissue transglutaminase. Clin Exp Immunol 125(2):216–221. https://doi.org/10.1046/j.1365-2249.2001.01584.x
Nakachi K, Powell M, Swift G, Amoroso MA, Ananieva-Jordanova R, Arnold C, Sanders J, Furmaniak J, Rees Smith B (2004) Epitopes recognised by tissue transglutaminase antibodies in coeliac disease. J Autoimmun 22(1):53–63
Sblattero D, Florian F, Azzoni E, Zyla T, Park M, Baldas V, Not T, Ventura A, Bradbury A, Marzari R (2002) The analysis of the fine specificity of celiac disease antibodies using tissue transglutaminase fragments. Eur J Biochem 269(21):5175–5181. https://doi.org/10.1046/j.1432-1033.2002.03215.x
Di Niro R, Ferrara F, Not T, Bradbury AR, Chirdo F, Marzari R, Sblattero D (2005) Characterizing monoclonal antibody epitopes by filtered gene fragment phage display. Biochem J 388(Pt 3):889–894. https://doi.org/10.1042/bj20041983
Király R, Vecsei Z, Deményi T, Korponay-Szabó IR, Fésüs L (2006) Coeliac auto-antibodies can enhance transamidating and inhibit GTPase activity of tissue transglutaminase: dependence on reaction environment and enzyme fitness. J Autoimmun 26(4):278–287. https://doi.org/10.1016/j.jaut.2006.03.002
Simon-Vecsei Z, Király R, Bagossi P, Tóth B, Dahlbom I, Caja S, Csosz É, Lindfors K, Sblattero D, Nemes É, Mäki M, Fésüs L, Korponay-Szabó IR (2012) A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects. Proc Natl Acad Sci USA 109(2):431–436. https://doi.org/10.1073/pnas.1107811108
Di Pisa M, Buccato P, Sabatino G, Real Fernández F, Berti B, Cocola F, Papini AM, Rovero P (2014) Epitope mapping of the N-terminal portion of tissue transglutaminase protein antigen to identify linear epitopes in celiac disease. J Pept Sci 20(9):689–695. https://doi.org/10.1002/psc.2650
Chen X, Hnida K, Graewert MA, Andersen JT, Iversen R, Tuukkanen A, Svergun D, Sollid LM (2015) Structural basis for antigen recognition by transglutaminase 2-specific auto-antibodies in celiac disease. J Biol Chem 290(35):21365–21375. https://doi.org/10.1074/jbc.M115.669895
Iversen R, Di Niro R, Stamnaes J, Lundin KE, Wilson PC, Sollid LM (2013) Transglutaminase 2-specific autoantibodies in celiac disease target clustered, N-terminal epitopes not displayed on the surface of cells. J Immunol 190(12):5981–5991. https://doi.org/10.4049/jimmunol.1300183
Byrne G, Ryan F, Jackson J, Feighery C, Kelly J (2007) Mutagenesis of the catalytic triad of tissue transglutaminase abrogates coeliac disease serum IgA autoantibody binding. Gut 56(3):336-41. https://doi.org/https://doi.org/10.1136/gut.2006.092908
Telci D, Griffin M (2006) Tissue transglutaminase (TG2)—a wound response enzyme. Front Biosci 11:867–882
Caputo I, Barone MV, Martucciello S, Lepretti M, Esposito C (2009) Tissue transglutaminase in celiac disease: role of autoantibodies. Amino Acids 36(4):693–699. https://doi.org/10.1007/s00726-008-0120-z
Halttunen T, Mäki M (1999) Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology 116(3):566–572
Esposito C, Paparo F, Caputo I, Rossi M, Maglio M, Sblattero D, Not T, Porta R, Auricchio S, Marzari R, Troncone R (2002) Anti-tissue transglutaminase antibodies from coeliac patients inhibit transglutaminase activity both in vitro and in situ. Gut 51(2):177–181. https://doi.org/10.1136/gut.51.2.177
Dieterich W, Trapp D, Esslinger B, Leidenberger M, Piper J, Hahn E, Schuppan D (2003) Auto-antibodies of patients with coeliac disease are insufficient to block tissue transglutaminase activity. Gut 52(11):1562–1566. https://doi.org/10.1136/gut.52.11.1562
Byrne G, Feighery C, Jackson J, Kelly J (2010) Coeliac disease auto-antibodies mediate significant inhibition of tissue transglutaminase. Clin Immunol 136(3):426–431. https://doi.org/10.1016/j.clim.2010.04.017
Anjum N, Baker PN, Robinson NJ, Aplin JD (2009) Maternal celiac disease auto-antibodies bind directly to syncytiotrophoblast and inhibit placental tissue transglutaminase activity. Reprod Biol Endocrinol 7:16. https://doi.org/10.1186/1477-7827-7-16
Di Niro R, Mesin L, Zheng NY, Stamnaes J, Morrissey M, Lee JH, Huang M, Iversen R, du Pré MF, Qiao SW, Lundin KE, Wilson PC, Sollid LM (2012) High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med 18(3):441–445. https://doi.org/10.1038/nm.2656
Myrsky E, Caja S, Simon-Vecsei Z, Korponay-Szabo IR, Nadalutti C, Collighan R, Mongeot A, Griffin M, Mäki M, Kaukinen K, Lindfors K (2009) Celiac disease IgA modulates vascular permeability in vitro through the activity of transglutaminase 2 and RhoA. Cell Mol Life Sci 66(20):3375–3385. https://doi.org/10.1007/s00018-009-0116-1
Caja S, Myrsky E, Korponay-Szabo IR, Nadalutti C, Sulic AM, Lavric M, Sblattero D, Marzari R, Collighan R, Mongeot A, Griffin M, Mäki M, Kaukinen K, Lindfors K (2010) Inhibition of transglutaminase 2 enzymatic activity ameliorates the anti-angiogenic effects of coeliac disease autoantibodies. Scand J Gastroenterol 45(4):421–427. https://doi.org/10.3109/00365520903540822
Kalliokoski S, Sulic AM, Korponay-Szabó IR, Szondy Z, Frias R, Perez MA, Martucciello S, Roivainen A, Pelliniemi LJ, Esposito C, Griffin M, Sblattero D, Mäki M, Kaukinen K, Lindfors K, Caja S (2013) Celiac disease-specific TG2-targeted auto-antibodies inhibit angiogenesis ex vivo and in vivo in mice by interfering with endothelial cell dynamics. PLoS One 8(6):e65887. https://doi.org/10.1371/journal.pone.0065887
Barone MV, Caputo I, Ribecco MT, Maglio M, Marzari R, Sblattero D, Troncone R, Auricchio S, Esposito C (2007) Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology 132(4):1245–1253. https://doi.org/10.1053/j.gastro.2007.01.030
Hnida K, Stamnaes J, du Pré MF, Mysling S, Jørgensen TJ, Sollid LM, Iversen R (2016) Epitope-dependent functional effects of celiac disease auto-antibodies on transglutaminase 2. J Biol Chem 291(49):25542–25552. https://doi.org/10.1074/jbc.M116.738161
Iversen R, Mysling S, Hnida K, Jørgensen TJ, Sollid LM (2014) Activity-regulating structural changes and autoantibody epitopes in transglutaminase 2 assessed by hydrogen/deuterium exchange. Proc Natl Acad Sci USA 111(48):17146–17151. https://doi.org/10.1073/pnas.1407457111
Caputo I, Barone MV, Lepretti M, Martucciello S, Nista I, Troncone R, Auricchio S, Sblattero D, Esposito C (2010) Celiac anti-tissue transglutaminase antibodies interfere with the uptake of alpha gliadin peptide 31–43 but not of peptide 57–68 by epithelial cells. Biochim Biophys Acta 9:717–727. https://doi.org/10.1016/j.bbadis.2010.05.010
Teesalu K, Panarina M, Uibo O, Uibo R, Utt M (2012) Autoantibodies from patients with celiac disease inhibit transglutaminase 2 binding to heparin/heparan sulfate and interfere with intestinal epithelial cell adhesion. Amino Acids 42(2–3):1055–1064. https://doi.org/10.1007/s00726-011-1020-1
Myrsky E, Kaukinen K, Syrjänen M, Korponay-Szabó IR, Mäki M, Lindfors K (2008) Coeliac disease-specific autoantibodies targeted against transglutaminase 2 disturb angiogenesis. Clin Exp Immunol 152(1):111–119. https://doi.org/10.1111/j.1365-2249.2008.03600.x
Nanayakkara M, Kosova R, Lania G, Sarno M, Gaito A, Galatola M, Greco L, Cuomo M, Troncone R, Auricchio S, Auricchio R, Barone MV (2013) A celiac cellular phenotype, with altered LPP sub-cellular distribution, is inducible in controls by the toxic gliadin peptide P31-43. PLoS One 8(11):e79763. https://doi.org/10.1371/journal.pone.0079763
Paolella G, Lepretti M, Barone MV, Nanayakkara M, Di Zenzo M, Sblattero D, Auricchio S, Esposito C, Caputo I (2017) Celiac anti-type 2 transglutaminase antibodies induce differential effects in fibroblasts from celiac disease patients and from healthy subjects. Amino Acid 49(3):541–550. https://doi.org/10.1007/s00726-016-2307-z
Caputo I, Lepretti M, Secondo A, Martucciello S, Paolella G, Sblattero D, Barone MV, Esposito C (2013) Anti-tissue transglutaminase antibodies activate intracellular tissue transglutaminase by modulating cytosolic Ca2+ homeostasis. Amino Acids 44(1):251–260. https://doi.org/10.1007/s00726-011-1120-y
Caputo I, Secondo A, Lepretti M, Paolella G, Auricchio S, Barone MV, Esposito C (2012) Gliadin peptides induce tissue transglutaminase activation and ER-stress through Ca2+ mobilization in Caco-2 cells. PLoS One 7(9):e45209. https://doi.org/10.1371/journal.pone.0045209
Paolella G, Caputo I, Marabotti A, Lepretti M, Salzano AM, Scaloni A, Vitale M, Zambrano N, Sblattero D, Esposito C (2013) Celiac anti-type 2 transglutaminase antibodies induce phosphoproteome modification in intestinal epithelial Caco-2 cells. PLoS One 8(12):e84403. https://doi.org/10.1371/journal.pone.0084403
Myrsky E, Syrjänen M, Korponay-Szabo IR, Mäki M, Kaukinen K, Lindfors K (2009) Altered small-bowel mucosal vascular network in untreated coeliac disease. Scand J Gastroenterol 44(2):162–167. https://doi.org/10.1080/00365520802400875
Martucciello S, Lavric M, Toth B, Korponay-Szabo I, Nadalutti C, Myrsky E, Rauhavirta T, Esposito C, Sulic AM, Sblattero D, Marzari R, Mäki M, Kaukinen K, Lindfors K, Caja S (2012) RhoB is associated with the anti-angiogenic effects of celiac patient transglutaminase 2-targeted auto-antibodies. J Mol Med (Berl) 90(7):817–826. https://doi.org/10.1007/s00109-011-0853-0
Lee YJ, Jung SH, Kim SH, Kim MS, Lee S, Hwang J, Kim SY, Kim YM, Ha KS (2016) Essential role of transglutaminase 2 in vascular endothelial growth factor-induced vascular leakage in the retina of diabetic mice. Diabetes 65(8):2414–2428. https://doi.org/10.2337/db15-1594
Adini I, Rabinovitz I, Sun JF, Prendergast GC, Benjamin LE (2003) Rho B controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev 17(21):2721–2732. https://doi.org/10.1101/gad.1134603
Nadalutti CA, Korponay-Szabo IR, Kaukinen K, Griffin M, Mäki M, Lindfors K (2014) Celiac disease patient IgA antibodies induce endothelial adhesion and cell polarization defects via extracellular transglutaminase 2. Cell Mol Life Sci 71(7):1315–1326. https://doi.org/10.1007/s00018-013-1455-5
Moss SF, Attia L, Scholes JV, Walters JR, Holt PR (1996) Increased small intestinal apoptosis in coeliac disease. Gut 39(6):811–817
Ciccocioppo R, Di Sabatino A, Parroni R, Muzi P, D’Alò S, Ventura T, Pistoia MA, Cifone MG, Corazza GR (2001) Increased enterocyte apoptosis and Fas-Fas ligand system in celiac disease. Am J Clin Pathol 115(4):494–503. https://doi.org/10.1309/uv54-bhp3-a66b-0qud
Hoffmanová I, Sánchez D, Hábová V, Anděl M, Tučková L, Tlaskalová-Hogenová H (2015) Serological markers of enterocyte damage and apoptosis in patients with celiac disease, autoimmune diabetes mellitus and diabetes mellitus type 2. Physiol Res 64(4):537–546
Kalliokoski S, Piqueras VO, Frías R, Sulic AM, Määttä JA, Kähkönen N, Viiri K, Huhtala H, Pasternack A, Laurila K, Sblattero D, Korponay-Szabó IR, Mäki M, Caja S, Kaukinen K, Lindfors K (2017) Transglutaminase 2-specific coeliac disease auto-antibodies induce morphological changes and signs of inflammation in the small-bowel mucosa of mice. Amino Acids 49(3):529–540. https://doi.org/10.1007/s00726-016-2306-0
Sóñora C, Calo G, Fraccaroli L, Pérez-Leirós C, Hernández A, Ramhorst R (2014) Tissue transglutaminase on trophoblast cells as a possible target of auto-antibodies contributing to pregnancy complications in celiac patients. Am J Reprod Immunol 72(5):485–495. https://doi.org/10.1111/aji.12290
Cervio E, Volta U, Verri M, Boschi F, Pastoris O, Granito A, Barbara G, Parisi C, Felicani C, Tonini M, De Giorgio R (2007) Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology 133(1):195–206. https://doi.org/10.1053/j.gastro.2007.04.070
Rauhavirta T, Qiao SW, Jiang Z, Myrsky E, Loponen J, Korponay-Szabó IR, Salovaara H, Garcia-Horsman JA, Venäläinen J, Männistö PT, Collighan R, Mongeot A, Griffin M, Mäki M, Kaukinen K, Lindfors K (2011) Epithelial transport and deamidation of gliadin peptides: a role for coeliac disease patient immunoglobulin A. Clin Exp Immunol 164(1):127–136. https://doi.org/10.1111/j.1365-2249.2010.04317.x
Paolella G, Lepretti M, Martucciello S, Nanayakkara M, Auricchio S, Esposito C, Barone MV, Caputo I (2018) The toxic alpha-gliadin peptide 31-43 enters cells without a surface membrane receptor. Cell biol Intern 42(1):112–120. https://doi.org/10.1002/cbin.10874
Freitag T, Schulze-Koops H, Niedobitek G, Melino G, Schuppan D (2004) The role of the immune response against tissue transglutaminase in the pathogenesis of coeliac disease. Autoimmun Rev 3(2):13–20. https://doi.org/10.1016/s1568-9972(03)00054-5
Di Niro R, Sblattero D, Florian F, Stebel M, Zentilin L, Giacca M, Villanacci V, Galletti A, Not T, Ventura A, Marzari R (2008) Anti-idiotypic response in mice expressing human auto-antibodies. Mol Immunol 45(6):1782–1791. https://doi.org/10.1016/j.molimm.2007.09.025
Vangone A, Abdel-Azeim S, Caputo I, Sblattero D, Di Niro R, Cavallo L, Oliva R (2014) Structural basis for the recognition in an idiotype-anti-idiotype antibody complex related to celiac disease. PLoS One 9(7):e102839. https://doi.org/10.1371/journal.pone.0102839
Tzioufas AG, Routsias JG (2010) Idiotype, anti-idiotype network of autoantibodies: pathogenetic considerations and clinical application. Autoimmun Rev 9:631–633
Kalliokoski S, Caja S, Frias R, Laurila K, Koskinen O, Niemelä O, Mäki M, Kaukinen K, Korponay-Szabó IR, Lindfors K (2015) Injection of celiac disease patient sera or immunoglobulins to mice reproduces a condition mimicking early developing celiac disease. J Mol Med (Berl) 93(1):51–62. https://doi.org/10.1007/s00109-014-1204-8
Hoffmanová I, Sánchez D, Tučková L, Tlaskalová-Hogenová H (2018) Celiac disease and liver disorders: from putative pathogenesis to clinical implications. Nutrients 10(7):E892. https://doi.org/10.3390/nu10070892
Hadjivassiliou M, Mäki M, Sanders DS, Williamson CA, Grünewald RA, Woodroofe NM, Korponay-Szabó IR (2006) Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology 66:373–377. https://doi.org/10.1212/01.wnl.0000196480.55601.3a
Boscolo S, Lorenzon A, Sblattero D, Florian F, Stebel M, Marzari R, Not T, Aeschlimann D, Ventura A, Hadjivassiliou M, Tongiorgi E (2010) Anti transglutaminase antibodies cause ataxia in mice. PLoS One 5(3):e9698. https://doi.org/10.1371/journal.pone.0009698
Sugai E, Cherñavsky A, Pedreira S, Smecuol E, Vazquez H, Niveloni S, Mazure R, Mauriro E, Rabinovich GA, Bai JC (2002) Bone-specific antibodies in sera from patients with celiac disease: characterization and implications in osteoporosis. J Clin Immunol 22(6):353–362. https://doi.org/10.1023/A:1020786315956
Naiyer AJ, Shah J, Hernandez L, Kim SY, Ciaccio EJ, Cheng J, Manavalan S, Bhagat G, Green PH (2008) Tissue transglutaminase antibodies in individuals with celiac disease bind to thyroid follicles and extracellular matrix and may contribute to thyroid dysfunction. Thyroid 18(11):1171–1178. https://doi.org/10.1089/thy.2008.0110
Sategna-Guidetti C, Franco E, Martini S, Bobbio M (2004) Binding by serum IgA antibodies from patients with coeliac disease to monkey heart tissue. Scand J Gastroenterol 39(6):540–543. https://doi.org/10.1080/00365520410008764
Sane DC, Kontos JL, Greenberg CS (2007) Roles of transglutaminases in cardiac and vascular diseases. Front Biosci 12:2530–2545
Lauret E, Rodrigo L (2013) Celiac disease and auto-immune-associated conditions. Biomed Res Int 2013:127589. https://doi.org/10.1155/2013/127589
Collin P, Salmi TT, Hervonen K, Kaukinen K, Reunala T (2017) Dermatitis herpetiformis: a cutaneous manifestation of coeliac disease. Ann Med 49(1):23–31. https://doi.org/10.1080/07853890.2016.1222450
Hällström O (1989) Comparison of IgA-class reticulin and endomysium antibodies in coeliac disease and dermatitis herpetiformis. Gut 30:1225–1232
Salmi TT, Hervonen K, Laurila K, Collin P, Mäki M, Koskinen O, Huhtala H, Kaukinen K, Reunala T (2014) Small bowel transglutaminase 2-specific IgA deposits in dermatitis herpetiformis. Acta Derm Venereol 94:393–397. https://doi.org/10.2340/00015555-1764
Rose C, Armbruster FP, Ruppert J, Igl BW, Zillikens D, Shimanovich I (2009) Auto-antibodies against epidermal transglutaminase are a sensitive diagnostic marker in patients with dermatitis herpetiformis on a normal or gluten-free diet. J Am Acad Dermatol 61(1):39–43. https://doi.org/10.1016/j.jaad.2008.12.037
Reunala T, Salmi TT, Hervonen K (2015) Dermatitis herpetiformis: pathognomonic transglutaminase IgA deposits in the skin and excellent prognosis on a gluten-free diet. Acta Derm Venereol 95(8):917–922. https://doi.org/10.2340/00015555-2162
Marietta EV, Camilleri MJ, Castro LA, Krause PK, Pittelkow MR, Murray JA (2008) Transgluaminase auto-antibodies in dermatitis herpetiformis and celiac sprue. J Invest Dermatol 128:332–335. https://doi.org/10.1038/sj.jid.5701041
Jackson JR, Eaton WW, Cascella NG, Fasano A, Kelly DL (2012) Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatr Q 83(1):91–102. https://doi.org/10.1007/s11126-011-9186-y
Hadjivassiliou M, Aeschlimann P, Sanders DS, Mäki M, Kaukinen K, Grünewald RA, Bandmann O, Woodroofe N, Haddock G, Aeschlimann DP (2013) Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology 80(19):1740–1745. https://doi.org/10.1212/WNL.0b013e3182919070
Zis P, Rao DG, Sarrigiannis PG, Aeschlimann P, Aeschlimann DP, Sanders D, Grünewald RA, Hadjivassiliou M (2017) Transglutaminase 6 antibodies in gluten neuropathy. Dig Liver Dis 49(11):1196–1200. https://doi.org/10.1016/j.dld.2017.08.019
De Leo L, Aeschlimann D, Hadjivassiliou M, Aeschlimann P, Salce N, Vatta S, Ziberna F, Cozzi G, Martelossi S, Ventura A, Not T (2018) Anti-transglutaminase 6 antibody development in children with celiac disease correlates with duration of gluten exposure. J Pediatr Gastroenterol Nutr 66:64–68. https://doi.org/10.1097/MPG.0000000000001642
Stamnaes J, Dorum S, Fleckenstein B, Aeschlimann D, Sollid LM (2010) Gluten T cell epitope targeting by TG3 and TG6; implications for dermatitis herpetiformis and gluten ataxia. Amino Acids 39(5):1183–1191. https://doi.org/10.1007/s00726-010-0554-y
Collin P, Kaukinen K, Välimäki M, Salmi J (2002) Endocrinological disorders and celiac disease. Endocr Rev 23:464–483. https://doi.org/10.1210/er.2001-0035
Lampasona V, Bonfanti R, Bazzigaluppi E, Venerando A, Chiumello G, Bosi E, Bonifacio E (1999) Antibodies to tissue TGase C in type I diabetes. Diabetologia 42:1195–1198. https://doi.org/10.1007/s001250051291
Camarca ME, Mozzillo E, Nugnes R, Zito E, Falco M, Fattorusso V, Mobilia S, Buono P, Valerio G, Troncone R, Franzese A (2012) Celiac disease in type 1 diabetes mellitus. Ital J Pediatr 38:10. https://doi.org/10.1186/1824-7288-38-10
Cohn A, Sofia AM, Kupfer SS (2014) Type 1 diabetes and celiac disease: clinical overlap and new insights into disease pathogenesis. Curr Diab Rep 14(8):517. https://doi.org/10.1007/s11892-014-0517-x
Bao F, Yu L, Babu S, Wang T, Hoffenberg EJ, Rewers M, Eisenbarth GS (1999) One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase auto-antibodies. J Autoimmun 13(1):143–148. https://doi.org/10.1006/jaut.1999.0303
McGinty JW, Marré ML, Bajzik V, Piganelli JD, James EA (2015) T cell epitopes and post-translationally modified epitopes in type 1 diabetes. Curr Diab Rep 15(11):90. https://doi.org/10.1007/s11892-015-0657-7
Ch’ng CL, Biswas M, Benton A, Jones MK, Kingham JGC (2005) Prospective screening for coeliac disease in patients with Graves’ hyperthyroidism using anti-gliadin and tissue transglutaminase antibodies. Clin Endocrinol (Oxf) 62(3):303–306. https://doi.org/10.1111/j.1365-2265.2005.02214.x
Hadithi M, de Boer H, Meijer JW, Willekens F, Kerckhaert JA, Heijmans R, Peña AS, Stehouwer CD, Mulder CJ (2007) Coeliac disease in Dutch patients with Hashimoto’s thyroiditis and vice versa. World J Gastroenterol 13:1715–1722. https://doi.org/10.3748/wjg.v13.i11.1715
Elfström P, Montgomery SM, Kampe O, Ekbom A, Ludvigsson JF (2008) Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab 93:3915–3921. https://doi.org/10.1210/jc.2008-0798
Badenhoop K, Dieterich W, Segni M, Hofmann S, Hüfner M, Usadel KH, Hahn EG, Schuppan D (2001) HLA DQ2 and/or DQ8 is associated with celiac disease-specific auto-antibodies to tissue transglutaminase in families with thyroid autoimmunity. Am J Gastroenterol 96(5):1648–1649. https://doi.org/10.1111/j.1572-0241.2001.03821.x
Luft LM, Barr SG, Martin LO, Chan EKL, Fritzler MJ (2003) Auto-antibodies to tissue transglutaminase in Sjögren’s syndrome and related rheumatic diseases. J Rheumatol 30:2613–2619
Marai I, Shoenfeld Y, Bizzaro N, Villalta D, Doria A, Tonutti E, Tozzoli R (2004) IgA and IgG tissue transglutaminase antibodies in systemic lupus erythematosus. Lupus 13:241–244. https://doi.org/10.1191/0961203304lu1004oa
Lerner A, Prager K, Matthias T (2015) Transient anti TG2 auto-antibodies in systemic lupus erythematosus: a window to autoimmunity. Int J Celiac Dis 3:72–74. https://doi.org/10.12691/ijcd-3-2-11
Ferrara F, Quaglia S, Caputo I, Esposito C, Lepretti M, Pastore S, Giorgi R, Martelossi S, Dal Molin G, Di Toro N, Ventura A, Not T (2010) Anti-transglutaminase antibodies in non-coeliac children suffering from infectious diseases. Clin Exp Immunol 159(2):212–217. https://doi.org/10.1111/j.1365-2249.2009.04054.x
Roth EB, Stenberg P, Book C, Sjöberg K (2006) Antibodies against transglutaminases, peptidylarginine deiminase and citrulline in rheumatoid arthritis—new pathways to epitope spreading. Clin Exp Rheumatol 24:12–18
Dahan S, Shor DB, Comaneshter D, Tekes-Manova D, Shovman O, Amital H, Cohen AD (2016) All disease begins in the gut: celiac disease co-existence with SLE. Autoimmun Rev 15:848–853. https://doi.org/10.1016/j.autrev.2016.06.003
Picarelli A, Di Tola M, Sabbatella L, Vetrano S, Anania MC, Spadaro A, Sorgi ML, Taccari E (2003) Anti-tissue transglutaminase antibodies in arthritic patients: a disease-specific finding? Clin Chem 49(12):2091–2094. https://doi.org/10.1373/clinchem.2003.023234
Király R, Csosz E, Kurtán T, Antus S, Szigeti K, Simon-Vecsei Z, Korponay-Szabó IR, Keresztessy Z, Fésüs L (2009) Functional significance of five noncanonical Ca2+-binding sites of human transglutaminase 2 characterized by site-directed mutagenesis. FEBS J 276(23):7083–7096. https://doi.org/10.1111/j.1742-4658.2009.07420.x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martucciello, S., Paolella, G., Esposito, C. et al. Anti-type 2 transglutaminase antibodies as modulators of type 2 transglutaminase functions: a possible pathological role in celiac disease. Cell. Mol. Life Sci. 75, 4107–4124 (2018). https://doi.org/10.1007/s00018-018-2902-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2902-0