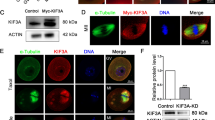
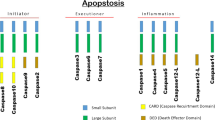
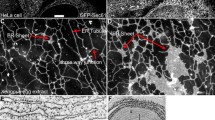
Abstract
Primary cilia are immotile organelles known for their roles in development and cell signaling. Defects in primary cilia result in a range of disorders named ciliopathies. Because this organelle can be found singularly on almost all cell types, its importance extends to most organ systems. As such, elucidating the importance of the primary cilium has attracted researchers from all biological disciplines. As the primary cilia field expands, caution is warranted in attributing biological defects solely to the function of this organelle, since many of these “ciliary” proteins are found at other sites in cells and likely have non-ciliary functions. Indeed, many, if not all, cilia proteins have locations and functions outside the primary cilium. Extraciliary functions are known to include cell cycle regulation, cytoskeletal regulation, and trafficking. Cilia proteins have been observed in the nucleus, at the Golgi apparatus, and even in immune synapses of T cells (interestingly, a non-ciliated cell). Given the abundance of extraciliary sites and functions, it can be difficult to definitively attribute an observed phenotype solely to defective cilia rather than to some defective extraciliary function or a combination of both. Thus, extraciliary sites and functions of cilia proteins need to be considered, as well as experimentally determined. Through such consideration, we will understand the true role of the primary cilium in disease as compared to other cellular processes’ influences in mediating disease (or through a combination of both). Here, we review a compilation of known extraciliary sites and functions of “cilia” proteins as a means to demonstrate the potential non-ciliary roles for these proteins.
Similar content being viewed by others
References
Ghosh A, Albers SV (2011) Assembly and function of the archaeal flagellum. Biochem Soc Trans 39(1):64–69. https://doi.org/10.1042/BST0390064
Pallen MJ, Matzke NJ (2006) From the origin of species to the origin of bacterial flagella. Nat Rev Microbiol 4(10):784–790. https://doi.org/10.1038/nrmicro1493
Mitchell DR (2007) The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv Exp Med Biol 607:130–140. https://doi.org/10.1007/978-0-387-74021-8_11
Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M (2011) Evolution: tracing the origins of centrioles, cilia, and flagella. J Cell Biol 194(2):165–175. https://doi.org/10.1083/jcb.201011152
Fisch C, Dupuis-Williams P (2011) Ultrastructure of cilia and flagella - back to the future. Biol Cell 103(6):249–270. https://doi.org/10.1042/BC20100139
Lindemann CB, Lesich KA (2016) Functional anatomy of the mammalian sperm flagellum. Cytoskeleton (Hoboken) 73(11):652–669. https://doi.org/10.1002/cm.21338
Tilley AE, Walters MS, Shaykhiev R, Crystal RG (2015) Cilia dysfunction in lung disease. Annu Rev Physiol 77:379–406. https://doi.org/10.1146/annurev-physiol-021014-071931
Lee L (2013) Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J Neurosci Res 91(9):1117–1132. https://doi.org/10.1002/jnr.23238
Satir P, Christensen ST (2007) Overview of structure and function of mammalian cilia. Annu Rev Physiol 69:377–400. https://doi.org/10.1146/annurev.physiol.69.040705.141236
Satir P, Christensen ST (2008) Structure and function of mammalian cilia. Histochem Cell Biol 129(6):687–693. https://doi.org/10.1007/s00418-008-0416-9
Singla V, Reiter JF (2006) The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313(5787):629–633. https://doi.org/10.1126/science.1124534
Marshall WF, Nonaka S (2006) Cilia: tuning in to the cell’s antenna. Curr Biol 16(15):R604–R614. https://doi.org/10.1016/j.cub.2006.07.012
Green JA, Mykytyn K (2014) Neuronal primary cilia: an underappreciated signaling and sensory organelle in the brain. Neuropsychopharmacology 39(1):244–245. https://doi.org/10.1038/npp.2013.203
Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK (2009) The primary cilium as a complex signaling center. Curr Biol 19(13):R526–R535. https://doi.org/10.1016/j.cub.2009.05.025
Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426(6962):83–87. https://doi.org/10.1038/nature02061
Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151(3):709–718
Menco BP (1984) Ciliated and microvillous structures of rat olfactory and nasal respiratory epithelia. A study using ultra-rapid cryo-fixation followed by freeze-substitution or freeze-etching. Cell Tissue Res 235(2):225–241
Jenkins PM, McEwen DP, Martens JR (2009) Olfactory cilia: linking sensory cilia function and human disease. Chem Sens 34(5):451–464. https://doi.org/10.1093/chemse/bjp020
Pennekamp P, Menchen T, Dworniczak B, Hamada H (2015) Situs inversus and ciliary abnormalities: 20 years later, what is the connection? Cilia 4(1):1. https://doi.org/10.1186/s13630-014-0010-9
Dobell C (1932) Antony van Leeuwenhoek and his “Little animals”; being some account of the father of protozoology and bacteriology and his multifarious discoveries in these disciplines. Harcourt, Brace and Company, New York
Zimmermann K (1898) Beitrage zur kenntniss einiger drusen und epihtelien. Arch Mikrosk Anat 52:552–706
Sorokin SP (1968) Centriole formation and ciliogenesis. Aspen Emphysema Conf 11:213–216
Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL (1993) A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA 90(12):5519–5523
Kozminski KG, Beech PL, Rosenbaum JL (1995) The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol 131(6 Pt 1):1517–1527
Hildebrandt F, Benzing T, Katsanis N (2011) Ciliopathies. N Engl J Med 364(16):1533–1543. https://doi.org/10.1056/NEJMra1010172
Mitchison HM, Valente EM (2017) Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol 241(2):294–309. https://doi.org/10.1002/path.4843
Waters AM, Beales PL (2011) Ciliopathies: an expanding disease spectrum. Pediatr Nephrol 26(7):1039–1056. https://doi.org/10.1007/s00467-010-1731-7
Huber C, Cormier-Daire V (2012) Ciliary disorder of the skeleton. Am J Med Genet C Semin Med Genet 160C(3):165–174. https://doi.org/10.1002/ajmg.c.31336
Jeune M, Beraud C, Carron R (1955) Asphyxiating thoracic dystrophy with familial characteristics. Arch Fr Pediatr 12(8):886–891
Parelkar SV, Kapadnis SP, Sanghvi BV, Joshi PB, Mundada D, Oak SN (2013) Meckel–Gruber syndrome: a rare and lethal anomaly with review of literature. J Pediatr Neurosci 8(2):154–157. https://doi.org/10.4103/1817-1745.117855
Suspitsin EN, Imyanitov EN (2016) Bardet-Biedl syndrome. Mol Syndromol 7(2):62–71. https://doi.org/10.1159/000445491
Joubert M, Eisenring JJ, Robb JP, Andermann F (1969) Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology 19(9):813–825
Boltshauser E, Isler W (1977) Joubert syndrome: episodic hyperpnea, abnormal eye movements, retardation and ataxia, associated with dysplasia of the cerebellar vermis. Neuropadiatrie 8(1):57–66. https://doi.org/10.1055/s-0028-1091505
Valente EM, Dallapiccola B, Bertini E (2013) Joubert syndrome and related disorders. Handb Clin Neurol 113:1879–1888. https://doi.org/10.1016/B978-0-444-59565-2.00058-7
Romani M, Micalizzi A, Valente EM (2013) Joubert syndrome: congenital cerebellar ataxia with the molar tooth. Lancet Neurol 12(9):894–905. https://doi.org/10.1016/S1474-4422(13)70136-4
Brancati F, Dallapiccola B, Valente EM (2010) Joubert syndrome and related disorders. Orphanet J Rare Dis 5:20. https://doi.org/10.1186/1750-1172-5-20
Ben-Salem S, Al-Shamsi AM, Gleeson JG, Ali BR, Al-Gazali L (2014) Mutation spectrum of Joubert syndrome and related disorders among Arabs. Hum Genome Var 1:14020. https://doi.org/10.1038/hgv.2014.20
Hua K, Bourgeois JR, Ferland RJ (2017) Joubert syndrome. Elsevier, Reference module in neuroscience and biobehavioral psychology. https://doi.org/10.1016/B978-0-12-809324-5.01953-2
Bachmann-Gagescu R (2014) Genetic complexity of ciliopathies and novel genes identification. Med Sci (Paris) 30(11):1011–1023. https://doi.org/10.1051/medsci/20143011016
Cardenas-Rodriguez M, Badano JL (2009) Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet 151C(4):263–280. https://doi.org/10.1002/ajmg.c.30227
Badano JL, Mitsuma N, Beales PL, Katsanis N (2006) The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genom Hum Genet 7:125–148. https://doi.org/10.1146/annurev.genom.7.080505.115610
Bader I, Decker E, Mayr JA, Lunzer V, Koch J, Boltshauser E, Sperl W, Pietsch P, Ertl-Wagner B, Bolz H, Bergmann C, Rittinger O (2016) MKS1 mutations cause Joubert syndrome with agenesis of the corpus callosum. Eur J Med Genet 59(8):386–391. https://doi.org/10.1016/j.ejmg.2016.06.007
Irfanullah KS, Ullah I, Nasir A, Meijer CA, Laurense-Bik M, den Dunnen JT, Ruivenkamp CA, Hoffer MJ, Santen GW, Ahmad W (2016) Hypomorphic MKS1 mutation in a Pakistani family with mild Joubert syndrome and atypical features: expanding the phenotypic spectrum of MKS1-related ciliopathies. Am J Med Genet A 170(12):3289–3293. https://doi.org/10.1002/ajmg.a.37934
Romani M, Micalizzi A, Kraoua I, Dotti MT, Cavallin M, Sztriha L, Ruta R, Mancini F, Mazza T, Castellana S, Hanene B, Carluccio MA, Darra F, Mate A, Zimmermann A, Gouider-Khouja N, Valente EM (2014) Mutations in B9D1 and MKS1 cause mild Joubert syndrome: expanding the genetic overlap with the lethal ciliopathy Meckel syndrome. Orphanet J Rare Dis 9:72. https://doi.org/10.1186/1750-1172-9-72
Slaats GG, Isabella CR, Kroes HY, Dempsey JC, Gremmels H, Monroe GR, Phelps IG, Duran KJ, Adkins J, Kumar SA, Knutzen DM, Knoers NV, Mendelsohn NJ, Neubauer D, Mastroyianni SD, Vogt J, Worgan L, Karp N, Bowdin S, Glass IA, Parisi MA, Otto EA, Johnson CA, Hildebrandt F, van Haaften G, Giles RH, Doherty D (2016) MKS1 regulates ciliary INPP5E levels in Joubert syndrome. J Med Genet 53(1):62–72. https://doi.org/10.1136/jmedgenet-2015-103250
Akizu N, Silhavy JL, Rosti RO, Scott E, Fenstermaker AG, Schroth J, Zaki MS, Sanchez H, Gupta N, Kabra M, Kara M, Ben-Omran T, Rosti B, Guemez-Gamboa A, Spencer E, Pan R, Cai N, Abdellateef M, Gabriel S, Halbritter J, Hildebrandt F, van Bokhoven H, Gunel M, Gleeson JG (2014) Mutations in CSPP1 lead to classical Joubert syndrome. Am J Hum Genet 94(1):80–86. https://doi.org/10.1016/j.ajhg.2013.11.015
Shaheen R, Shamseldin HE, Loucks CM, Seidahmed MZ, Ansari S, Ibrahim Khalil M, Al-Yacoub N, Davis EE, Mola NA, Szymanska K, Herridge W, Chudley AE, Chodirker BN, Schwartzentruber J, Majewski J, Katsanis N, Poizat C, Johnson CA, Parboosingh J, Boycott KM, Innes AM, Alkuraya FS (2014) Mutations in CSPP1, encoding a core centrosomal protein, cause a range of ciliopathy phenotypes in humans. Am J Hum Genet 94(1):73–79. https://doi.org/10.1016/j.ajhg.2013.11.010
Tuz K, Bachmann-Gagescu R, O’Day DR, Hua K, Isabella CR, Phelps IG, Stolarski AE, O’Roak BJ, Dempsey JC, Lourenco C, Alswaid A, Bonnemann CG, Medne L, Nampoothiri S, Stark Z, Leventer RJ, Topcu M, Cansu A, Jagadeesh S, Done S, Ishak GE, Glass IA, Shendure J, Neuhauss SC, Haldeman-Englert CR, Doherty D, Ferland RJ (2014) Mutations in CSPP1 cause primary cilia abnormalities and Joubert syndrome with or without Jeune asphyxiating thoracic dystrophy. Am J Hum Genet 94(1):62–72. https://doi.org/10.1016/j.ajhg.2013.11.019
Ben-Omran T, Alsulaiman R, Kamel H, Shaheen R, Alkuraya FS (2015) Intrafamilial clinical heterogeneity of CSPP1-related ciliopathy. Am J Med Genet A 167A(10):2478–2480. https://doi.org/10.1002/ajmg.a.37175
Kammenga JE (2017) The background puzzle: how identical mutations in the same gene lead to different disease symptoms. FEBS J. https://doi.org/10.1111/febs.14080
Ramsbottom S, Miles C, Sayer J (2015) Murine Cep290 phenotypes are modified by genetic backgrounds and provide an impetus for investigating disease modifier alleles. F1000Res 4:590–2480. https://doi.org/10.12688/f1000research.6959.1
Ishikawa H, Marshall WF (2011) Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 12(4):222–234. https://doi.org/10.1038/nrm3085
Santos N, Reiter JF (2008) Building it up and taking it down: the regulation of vertebrate ciliogenesis. Dev Dyn 237(8):1972–1981. https://doi.org/10.1002/dvdy.21540
Avasthi P, Marshall WF (2012) Stages of ciliogenesis and regulation of ciliary length. Differentiation 83(2):S30–S42. https://doi.org/10.1016/j.diff.2011.11.015
Nozawa YI, Lin C, Chuang PT (2013) Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr Opin Genet Dev 23(4):429–437. https://doi.org/10.1016/j.gde.2013.04.008
Sasai N, Briscoe J (2012) Primary cilia and graded Sonic Hedgehog signaling. Wiley Interdiscip Rev Dev Biol 1(5):753–772. https://doi.org/10.1002/wdev.43
Albee AJ, Kwan AL, Lin H, Granas D, Stormo GD, Dutcher SK (2013) Identification of cilia genes that affect cell-cycle progression using whole-genome transcriptome analysis in Chlamydomonas reinhardtti. G3 (Bethesda) 3(6):979–991. https://doi.org/10.1534/g3.113.006338
Hsiao YC, Tong ZJ, Westfall JE, Ault JG, Page-McCaw PS, Ferland RJ (2009) Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Hum Mol Genet 18(20):3926–3941. https://doi.org/10.1093/hmg/ddp335
Kennah E, Ringrose A, Zhou LL, Esmailzadeh S, Qian H, Su MW, Zhou Y, Jiang X (2009) Identification of tyrosine kinase, HCK, and tumor suppressor, BIN1, as potential mediators of AHI-1 oncogene in primary and transformed CTCL cells. Blood 113(19):4646–4655. https://doi.org/10.1182/blood-2008-08-174037
Zhou LL, Zhao Y, Ringrose A, DeGeer D, Kennah E, Lin AE, Sheng G, Li XJ, Turhan A, Jiang X (2008) AHI-1 interacts with BCR-ABL and modulates BCR-ABL transforming activity and imatinib response of CML stem/progenitor cells. J Exp Med 205(11):2657–2671. https://doi.org/10.1084/jem.20072316
Esmailzadeh S, Jiang X (2011) AHI-1: a novel signaling protein and potential therapeutic target in human leukemia and brain disorders. Oncotarget 2(12):918–934. https://doi.org/10.18632/oncotarget.405
Jiang X, Hanna Z, Kaouass M, Girard L, Jolicoeur P (2002) Ahi-1, a novel gene encoding a modular protein with WD40-repeat and SH3 domains, is targeted by the Ahi-1 and Mis-2 provirus integrations. J Virol 76(18):9046–9059
Patzke S, Hauge H, Sioud M, Finne EF, Sivertsen EA, Delabie J, Stokke T, Aasheim HC (2005) Identification of a novel centrosome/microtubule-associated coiled-coil protein involved in cell-cycle progression and spindle organization. Oncogene 24(7):1159–1173. https://doi.org/10.1038/sj.onc.1208267
Asiedu M, Wu D, Matsumura F, Wei Q (2009) Centrosome/spindle pole-associated protein regulates cytokinesis via promoting the recruitment of MyoGEF to the central spindle. Mol Biol Cell 20(5):1428–1440. https://doi.org/10.1091/mbc.E08-01-0001
Zhu L, Wang Z, Wang W, Wang C, Hua S, Su Z, Brako L, Garcia-Barrio M, Ye M, Wei X, Zou H, Ding X, Liu L, Liu X, Yao X (2015) Mitotic protein CSPP1 interacts with CENP-H protein to coordinate accurate chromosome oscillation in mitosis. J Biol Chem 290(45):27053–27066. https://doi.org/10.1074/jbc.M115.658534
Sternemalm J, Russnes HG, Zhao X, Risberg B, Nord S, Caldas C, Borresen-Dale AL, Stokke T, Patzke S (2014) Nuclear CSPP1 expression defined subtypes of basal-like breast cancer. Br J Cancer 111(2):326–338. https://doi.org/10.1038/bjc.2014.297
Patzke S, Redick S, Warsame A, Murga-Zamalloa CA, Khanna H, Doxsey S, Stokke T (2010) CSPP is a ciliary protein interacting with Nephrocystin 8 and required for cilia formation. Mol Biol Cell 21(15):2555–2567. https://doi.org/10.1091/mbc.E09-06-0503
Jung KH, Noh JH, Kim JK, Eun JW, Bae HJ, Xie HJ, Chang YG, Kim MG, Park H, Lee JY, Nam SW (2012) HDAC2 overexpression confers oncogenic potential to human lung cancer cells by deregulating expression of apoptosis and cell cycle proteins. J Cell Biochem 113(6):2167–2177. https://doi.org/10.1002/jcb.24090
Kobayashi T, Nakazono K, Tokuda M, Mashima Y, Dynlacht BD, Itoh H (2017) HDAC2 promotes loss of primary cilia in pancreatic ductal adenocarcinoma. EMBO Rep 18(2):334–343. https://doi.org/10.15252/embr.201541922
Delaval B, Bright A, Lawson ND, Doxsey S (2011) The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol 13(4):461–468. https://doi.org/10.1038/ncb2202
Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M (2006) Defective planar cell polarity in polycystic kidney disease. Nat Genet 38(1):21–23. https://doi.org/10.1038/ng1701
Feng Y, Walsh CA (2004) Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron 44(2):279–293. https://doi.org/10.1016/j.neuron.2004.09.023
Hehnly H, Doxsey S (2014) Rab11 endosomes contribute to mitotic spindle organization and orientation. Dev Cell 28(5):497–507. https://doi.org/10.1016/j.devcel.2014.01.014
Kitagawa D, Kohlmaier G, Keller D, Strnad P, Balestra FR, Fluckiger I, Gonczy P (2011) Spindle positioning in human cells relies on proper centriole formation and on the microcephaly proteins CPAP and STIL. J Cell Sci 124(Pt 22):3884–3893. https://doi.org/10.1242/jcs.089888
Kim JC, Ou YY, Badano JL, Esmail MA, Leitch CC, Fiedrich E, Beales PL, Archibald JM, Katsanis N, Rattner JB, Leroux MR (2005) MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet-Biedl syndrome, is a novel centrosomal component required for cytokinesis. J Cell Sci 118(Pt 5):1007–1020. https://doi.org/10.1242/jcs.01676
Wood CR, Wang Z, Diener D, Zones JM, Rosenbaum J, Umen JG (2012) IFT proteins accumulate during cell division and localize to the cleavage furrow in Chlamydomonas. PLoS One 7(2):e30729. https://doi.org/10.1371/journal.pone.0030729
Bernabe-Rubio M, Andres G, Casares-Arias J, Fernandez-Barrera J, Rangel L, Reglero-Real N, Gershlick DC, Fernandez JJ, Millan J, Correas I, Miguez DG, Alonso MA (2016) Novel role for the midbody in primary ciliogenesis by polarized epithelial cells. J Cell Biol 214(3):259–273. https://doi.org/10.1083/jcb.201601020
Fry AM, O’Regan L, Sabir SR, Bayliss R (2012) Cell cycle regulation by the NEK family of protein kinases. J Cell Sci 125(Pt 19):4423–4433. https://doi.org/10.1242/jcs.111195
O’Regan L, Blot J, Fry AM (2007) Mitotic regulation by NIMA-related kinases. Cell Div 2:25. https://doi.org/10.1186/1747-1028-2-25
Mahjoub MR, Trapp ML, Quarmby LM (2005) NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol 16(12):3485–3489. https://doi.org/10.1681/ASN.2005080824
Shalom O, Shalva N, Altschuler Y, Motro B (2008) The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett 582(10):1465–1470. https://doi.org/10.1016/j.febslet.2008.03.036
Monroe GR, Kappen IF, Stokman MF, Terhal PA, van den Boogaard MH, Savelberg SM, van der Veken LT, van Es RJ, Lens SM, Hengeveld RC, Creton MA, Janssen NG, Mink van der Molen AB, Ebbeling MB, Giles RH, Knoers NV, van Haaften G (2016) Compound heterozygous NEK1 variants in two siblings with oral-facial-digital syndrome type II (Mohr syndrome). Eur J Hum Genet 24(12):1752–1760. https://doi.org/10.1038/ejhg.2016.103
Al-Jassar C, Andreeva A, Barnabas DD, McLaughlin SH, Johnson CM, Yu M, van Breugel M (2017) The ciliopathy-associated Cep104 protein interacts with Tubulin and Nek1 kinase. Structure 25(1):146–156. https://doi.org/10.1016/j.str.2016.11.014
Boisvieux-Ulrich E, Laine MC, Sandoz D (1989) In vitro effects of colchicine and nocodazole on ciliogenesis in quail oviduct. Biol Cell 67(1):67–79
Boisvieux-Ulrich E, Laine MC, Sandoz D (1989) In vitro effects of taxol on ciliogenesis in quail oviduct. J Cell Sci 92(Pt 1):9–20
Patzke S, Stokke T, Aasheim HC (2006) CSPP and CSPP-L associate with centrosomes and microtubules and differently affect microtubule organization. J Cell Physiol 209(1):199–210. https://doi.org/10.1002/jcp.20725
Das A, Dickinson DJ, Wood CC, Goldstein B, Slep KC (2015) Crescerin uses a TOG domain array to regulate microtubules in the primary cilium. Mol Biol Cell 26(23):4248–4264. https://doi.org/10.1091/mbc.E15-08-0603
Dacheux D, Roger B, Bosc C, Landrein N, Roche E, Chansel L, Trian T, Andrieux A, Papaxanthos-Roche A, Marthan R, Robinson DR, Bonhivers M (2015) Human FAM154A (SAXO1) is a microtubule-stabilizing protein specific to cilia and related structures. J Cell Sci 128(7):1294–1307. https://doi.org/10.1242/jcs.155143
McNally FJ, Vale RD (1993) Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell 75(3):419–429
Sharp DJ, Ross JL (2012) Microtubule-severing enzymes at the cutting edge. J Cell Sci 125(Pt 11):2561–2569. https://doi.org/10.1242/jcs.101139
Sharma N, Bryant J, Wloga D, Donaldson R, Davis RC, Jerka-Dziadosz M, Gaertig J (2007) Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J Cell Biol 178(6):1065–1079. https://doi.org/10.1083/jcb.200704021
Hu WF, Pomp O, Ben-Omran T, Kodani A, Henke K, Mochida GH, Yu TW, Woodworth MB, Bonnard C, Raj GS, Tan TT, Hamamy H, Masri A, Shboul M, Al Saffar M, Partlow JN, Al-Dosari M, Alazami A, Alowain M, Alkuraya FS, Reiter JF, Harris MP, Reversade B, Walsh CA (2014) Katanin p80 regulates human cortical development by limiting centriole and cilia number. Neuron 84(6):1240–1257. https://doi.org/10.1016/j.neuron.2014.12.017
Sanders AA, de Vrieze E, Alazami AM, Alzahrani F, Malarkey EB, Sorusch N, Tebbe L, Kuhns S, van Dam TJ, Alhashem A, Tabarki B, Lu Q, Lambacher NJ, Kennedy JE, Bowie RV, Hetterschijt L, van Beersum S, van Reeuwijk J, Boldt K, Kremer H, Kesterson RA, Monies D, Abouelhoda M, Roepman R, Huynen MH, Ueffing M, Russell RB, Wolfrum U, Yoder BK, van Wijk E, Alkuraya FS, Blacque OE (2015) KIAA0556 is a novel ciliary basal body component mutated in Joubert syndrome. Genome Biol 16:293. https://doi.org/10.1186/s13059-015-0858-z
Banks G, Lassi G, Hoerder-Suabedissen A, Tinarelli F, Simon MM, Wilcox A, Lau P, Lawson TN, Johnson S, Rutman A, Sweeting M, Chesham JE, Barnard AR, Horner N, Westerberg H, Smith LB, Molnar Z, Hastings MH, Hirst RA, Tucci V, Nolan PM (2017) A missense mutation in Katnal1 underlies behavioural, neurological and ciliary anomalies. Mol Psychiatry. https://doi.org/10.1038/mp.2017.54
Ververis A, Christodoulou A, Christoforou M, Kamilari C, Lederer CW, Santama N (2016) A novel family of katanin-like 2 protein isoforms (KATNAL2), interacting with nucleotide-binding proteins Nubp1 and Nubp2, are key regulators of different MT-based processes in mammalian cells. Cell Mol Life Sci 73(1):163–184. https://doi.org/10.1007/s00018-015-1980-5
Yan X, Zhu X (2013) Branched F-actin as a negative regulator of cilia formation. Exp Cell Res 319(2):147–151. https://doi.org/10.1016/j.yexcr.2012.08.009
Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE (2010) MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19(2):270–283. https://doi.org/10.1016/j.devcel.2010.07.009
Yin Y, Bangs F, Paton IR, Prescott A, James J, Davey MG, Whitley P, Genikhovich G, Technau U, Burt DW, Tickle C (2009) The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development 136(4):655–664. https://doi.org/10.1242/dev.028464
Roosing S, Hofree M, Kim S, Scott E, Copeland B, Romani M, Silhavy JL, Rosti RO, Schroth J, Mazza T, Miccinilli E, Zaki MS, Swoboda KJ, Milisa-Drautz J, Dobyns WB, Mikati MA, Incecik F, Azam M, Borgatti R, Romaniello R, Boustany RM, Clericuzio CL, D’Arrigo S, Stromme P, Boltshauser E, Stanzial F, Mirabelli-Badenier M, Moroni I, Bertini E, Emma F, Steinlin M, Hildebrandt F, Johnson CA, Freilinger M, Vaux KK, Gabriel SB, Aza-Blanc P, Heynen-Genel S, Ideker T, Dynlacht BD, Lee JE, Valente EM, Kim J, Gleeson JG (2015) Functional genome-wide siRNA screen identifies KIAA0586 as mutated in Joubert syndrome. Elife 4:e06602. https://doi.org/10.7554/eLife.06602
Bachmann-Gagescu R, Phelps IG, Dempsey JC, Sharma VA, Ishak GE, Boyle EA, Wilson M, Marques Lourenco C, Arslan M, Shendure J, Doherty D, University of Washington Center for Mendelian G (2015) KIAA0586 is mutated in Joubert syndrome. Hum Mutat 36(9):831–835. https://doi.org/10.1002/humu.22821
Stephen LA, Tawamie H, Davis GM, Tebbe L, Nurnberg P, Nurnberg G, Thiele H, Thoenes M, Boltshauser E, Uebe S, Rompel O, Reis A, Ekici AB, McTeir L, Fraser AM, Hall EA, Mill P, Daudet N, Cross C, Wolfrum U, Jamra RA, Davey MG, Bolz HJ (2015) TALPID3 controls centrosome and cell polarity and the human ortholog KIAA0586 is mutated in Joubert syndrome (JBTS23). Elife. https://doi.org/10.7554/eLife.08077
Alby C, Piquand K, Huber C, Megarbane A, Ichkou A, Legendre M, Pelluard F, Encha-Ravazi F, Abi-Tayeh G, Bessieres B, El Chehadeh-Djebbar S, Laurent N, Faivre L, Sztriha L, Zombor M, Szabo H, Failler M, Garfa-Traore M, Bole C, Nitschke P, Nizon M, Elkhartoufi N, Clerget-Darpoux F, Munnich A, Lyonnet S, Vekemans M, Saunier S, Cormier-Daire V, Attie-Bitach T, Thomas S (2015) Mutations in KIAA0586 cause lethal ciliopathies ranging from a hydrolethalus phenotype to short-Rib polydactyly syndrome. Am J Hum Genet 97(2):311–318. https://doi.org/10.1016/j.ajhg.2015.06.003
Malicdan MC, Vilboux T, Stephen J, Maglic D, Mian L, Konzman D, Guo J, Yildirimli D, Bryant J, Fischer R, Zein WM, Snow J, Vemulapalli M, Mullikin JC, Toro C, Solomon BD, Niederhuber JE, Program NCS, Gahl WA, Gunay-Aygun M (2015) Mutations in human homologue of chicken talpid3 gene (KIAA0586) cause a hybrid ciliopathy with overlapping features of Jeune and Joubert syndromes. J Med Genet 52(12):830–839. https://doi.org/10.1136/jmedgenet-2015-103316
Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464(7291):1048–1051. https://doi.org/10.1038/nature08895
Cao J, Shen Y, Zhu L, Xu Y, Zhou Y, Wu Z, Li Y, Yan X, Zhu X (2012) miR-129-3p controls cilia assembly by regulating CP110 and actin dynamics. Nat Cell Biol 14(7):697–706. https://doi.org/10.1038/ncb2512
Megaw R, Abu-Arafeh H, Jungnickel M, Mellough C, Gurniak C, Witke W, Zhang W, Khanna H, Mill P, Dhillon B, Wright AF, Lako M, Ffrench-Constant C (2017) Gelsolin dysfunction causes photoreceptor loss in induced pluripotent cell and animal retinitis pigmentosa models. Nat Commun 8(1):271. https://doi.org/10.1038/s41467-017-00111-8
Gakovic M, Shu X, Kasioulis I, Carpanini S, Moraga I, Wright AF (2011) The role of RPGR in cilia formation and actin stability. Hum Mol Genet 20(24):4840–4850. https://doi.org/10.1093/hmg/ddr423
Rao KN, Li L, Zhang W, Brush RS, Rajala RV, Khanna H (2016) Loss of human disease protein retinitis pigmentosa GTPase regulator (RPGR) differentially affects rod or cone-enriched retina. Hum Mol Genet 25(7):1345–1356. https://doi.org/10.1093/hmg/ddw017
Zhang C, Zhang W, Lu Y, Yan X, Yan X, Zhu X, Liu W, Yang Y, Zhou T (2016) NudC regulates actin dynamics and ciliogenesis by stabilizing cofilin 1. Cell Res 26(2):239–253. https://doi.org/10.1038/cr.2015.152
Jones TJ, Adapala RK, Geldenhuys WJ, Bursley C, AbouAlaiwi WA, Nauli SM, Thodeti CK (2012) Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of hsp27 dependent actin cytoskeletal organization. J Cell Physiol 227(1):70–76. https://doi.org/10.1002/jcp.22704
Ravanelli AM, Klingensmith J (2011) The actin nucleator Cordon-bleu is required for development of motile cilia in zebrafish. Dev Biol 350(1):101–111. https://doi.org/10.1016/j.ydbio.2010.11.023
Rao Y, Hao R, Wang B, Yao TP (2014) A Mec17-Myosin II effector axis coordinates microtubule acetylation and actin dynamics to control primary cilium biogenesis. PLoS One 9(12):e114087. https://doi.org/10.1371/journal.pone.0114087
Hong H, Kim J, Kim J (2015) Myosin heavy chain 10 (MYH10) is required for centriole migration during the biogenesis of primary cilia. Biochem Biophys Res Commun 461(1):180–185. https://doi.org/10.1016/j.bbrc.2015.04.028
Assis LH, Silva-Junior RM, Dolce LG, Alborghetti MR, Honorato RV, Nascimento AF, Melo-Hanchuk TD, Trindade DM, Tonoli CC, Santos CT, Oliveira PS, Larson RE, Kobarg J, Espreafico EM, Giuseppe PO, Murakami MT (2017) The molecular motor Myosin Va interacts with the cilia-centrosomal protein RPGRIP1L. Sci Rep 7:43692. https://doi.org/10.1038/srep43692
Doering JE, Kane K, Hsiao YC, Yao C, Shi B, Slowik AD, Dhagat B, Scott DD, Ault JG, Page-McCaw PS, Ferland RJ (2008) Species differences in the expression of Ahi1, a protein implicated in the neurodevelopmental disorder Joubert syndrome, with preferential accumulation to stigmoid bodies. J Comp Neurol 511(2):238–256. https://doi.org/10.1002/cne.21824
Spampinato MV, Kraas J, Maria BL, Walton ZJ, Rumboldt Z (2008) Absence of decussation of the superior cerebellar peduncles in patients with Joubert syndrome. Am J Med Genet A 146A(11):1389–1394. https://doi.org/10.1002/ajmg.a.32282
Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, Shugart YY, Ruvolo M, Walsh CA (2004) Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 36(9):1008–1013. https://doi.org/10.1038/ng1419
Yeyati PL, Schiller R, Mali G, Kasioulis I, Kawamura A, Adams IR, Playfoot C, Gilbert N, van Heyningen V, Wills J, von Kriegsheim A, Finch A, Sakai J, Schofield CJ, Jackson IJ, Mill P (2017) KDM3A coordinates actin dynamics with intraflagellar transport to regulate cilia stability. J Cell Biol 216(4):999–1013. https://doi.org/10.1083/jcb.201607032
Avasthi P, Onishi M, Karpiak J, Yamamoto R, Mackinder L, Jonikas MC, Sale WS, Shoichet B, Pringle JR, Marshall WF (2014) Actin is required for IFT regulation in Chlamydomonas reinhardtii. Curr Biol 24(17):2025–2032. https://doi.org/10.1016/j.cub.2014.07.038
May-Simera HL, Gumerson JD, Gao C, Campos M, Cologna SM, Beyer T, Boldt K, Kaya KD, Patel N, Kretschmer F, Kelley MW, Petralia RS, Davey MG, Li T (2016) Loss of MACF1 abolishes ciliogenesis and disrupts apicobasal polarity establishment in the retina. Cell Rep 17(5):1399–1413. https://doi.org/10.1016/j.celrep.2016.09.089
Mizuno H, Watanabe N (2012) mDia1 and formins: screw cap of the actin filament. Biophysics (Nagoya-shi) 8:95–102. https://doi.org/10.2142/biophysics.8.95
Li F, Higgs HN (2003) The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol 13(15):1335–1340
Pan J, Lordier L, Meyran D, Rameau P, Lecluse Y, Kitchen-Goosen S, Badirou I, Mokrani H, Narumiya S, Alberts AS, Vainchenker W, Chang Y (2014) The formin DIAPH1 (mDia1) regulates megakaryocyte proplatelet formation by remodeling the actin and microtubule cytoskeletons. Blood 124(26):3967–3977. https://doi.org/10.1182/blood-2013-12-544924
Bartolini F, Ramalingam N, Gundersen GG (2012) Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol Biol Cell 23(20):4032–4040. https://doi.org/10.1091/mbc.E12-05-0338
Kato T, Watanabe N, Morishima Y, Fujita A, Ishizaki T, Narumiya S (2001) Localization of a mammalian homolog of diaphanous, mDia1, to the mitotic spindle in HeLa cells. J Cell Sci 114(Pt 4):775–784
Zhang Y, Wang F, Niu YJ, Liu HL, Rui R, Cui XS, Kim NH, Sun SC (2015) Formin mDia1, a downstream molecule of FMNL1, regulates Profilin1 for actin assembly and spindle organization during mouse oocyte meiosis. Biochim Biophys Acta 1853 2:317–327. https://doi.org/10.1016/j.bbamcr.2014.11.005
Rundle DR, Gorbsky G, Tsiokas L (2004) PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: role of mDia1 IN PKD2 localization to mitotic spindles. J Biol Chem 279(28):29728–29739. https://doi.org/10.1074/jbc.M400544200
Mostowy S, Cossart P (2012) Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol 13(3):183–194. https://doi.org/10.1038/nrm3284
Kim MS, Froese CD, Xie H, Trimble WS (2016) Immunofluorescent staining of septins in primary cilia. Methods Cell Biol 136:269–283. https://doi.org/10.1016/bs.mcb.2016.03.015
Ghossoub R, Hu Q, Failler M, Rouyez MC, Spitzbarth B, Mostowy S, Wolfrum U, Saunier S, Cossart P, Jamesnelson W, Benmerah A (2013) Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J Cell Sci 126(Pt 12):2583–2594. https://doi.org/10.1242/jcs.111377
Fliegauf M, Kahle A, Haffner K, Zieger B (2014) Distinct localization of septin proteins to ciliary sub-compartments in airway epithelial cells. Biol Chem 395(2):151–156. https://doi.org/10.1515/hsz-2013-0252
Schou KB, Pedersen LB, Christensen ST (2015) Ins and outs of GPCR signaling in primary cilia. EMBO Rep 16(9):1099–1113. https://doi.org/10.15252/embr.201540530
Lechtreck KF (2015) IFT-cargo interactions and protein transport in Cilia. Trends Biochem Sci 40(12):765–778. https://doi.org/10.1016/j.tibs.2015.09.003
Lechtreck KF (2016) Methods for studying movement of molecules within Cilia. Methods Mol Biol 1454:83–96. https://doi.org/10.1007/978-1-4939-3789-9_6
Madhivanan K, Aguilar RC (2014) Ciliopathies: the trafficking connection. Traffic 15(10):1031–1056. https://doi.org/10.1111/tra.12195
Taschner M, Lorentzen E (2016) The intraflagellar transport machinery. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a028092
Mourao A, Christensen ST, Lorentzen E (2016) The intraflagellar transport machinery in ciliary signaling. Curr Opin Struct Biol 41:98–108. https://doi.org/10.1016/j.sbi.2016.06.009
Hsiao YC, Tuz K, Ferland RJ (2012) Trafficking in and to the primary cilium. Cilia 1(1):4. https://doi.org/10.1186/2046-2530-1-4
Kleyn PW, Fan W, Kovats SG, Lee JJ, Pulido JC, Wu Y, Berkemeier LR, Misumi DJ, Holmgren L, Charlat O, Woolf EA, Tayber O, Brody T, Shu P, Hawkins F, Kennedy B, Baldini L, Ebeling C, Alperin GD, Deeds J, Lakey ND, Culpepper J, Chen H, Glucksmann-Kuis MA, Carlson GA, Duyk GM, Moore KJ (1996) Identification and characterization of the mouse obesity gene tubby: a member of a novel gene family. Cell 85(2):281–290
Sun X, Haley J, Bulgakov OV, Cai X, McGinnis J, Li T (2012) Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia 1(1):21. https://doi.org/10.1186/2046-2530-1-21
Short B (2017) Tubby proteins prove their adaptability. J Cell Biol 216(3):527. https://doi.org/10.1083/jcb.201702052
Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK (2010) TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev 24(19):2180–2193. https://doi.org/10.1101/gad.1966210
Mukhopadhyay S, Jackson PK (2011) The tubby family proteins. Genome Biol 12(6):225. https://doi.org/10.1186/gb-2011-12-6-225
Badgandi HB, Hwang SH, Shimada IS, Loriot E, Mukhopadhyay S (2017) Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol 216(3):743–760. https://doi.org/10.1083/jcb.201607095
Rohatgi R, Snell WJ (2010) The ciliary membrane. Curr Opin Cell Biol 22(4):541–546. https://doi.org/10.1016/j.ceb.2010.03.010
Nakatsu F (2015) A phosphoinositide code for primary cilia. Dev Cell 34(4):379–380. https://doi.org/10.1016/j.devcel.2015.08.008
Park J, Lee N, Kavoussi A, Seo JT, Kim CH, Moon SJ (2015) Ciliary phosphoinositide regulates ciliary protein trafficking in drosophila. Cell Rep 13(12):2808–2816. https://doi.org/10.1016/j.celrep.2015.12.009
Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL (1992) The Lowe’s oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358(6383):239–242. https://doi.org/10.1038/358239a0
Zhang X, Jefferson AB, Auethavekiat V, Majerus PW (1995) The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci USA 92(11):4853–4856
Coon BG, Hernandez V, Madhivanan K, Mukherjee D, Hanna CB, Barinaga-Rementeria Ramirez I, Lowe M, Beales PL, Aguilar RC (2012) The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum Mol Genet 21(8):1835–1847. https://doi.org/10.1093/hmg/ddr615
Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y (2012) OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Hum Mol Genet 21(15):3333–3344. https://doi.org/10.1093/hmg/dds163
Rbaibi Y, Cui S, Mo D, Carattino M, Rohatgi R, Satlin LM, Szalinski CM, Swanhart LM, Folsch H, Hukriede NA, Weisz OA (2012) OCRL1 modulates cilia length in renal epithelial cells. Traffic 13(9):1295–1305. https://doi.org/10.1111/j.1600-0854.2012.01387.x
Prosseda PP, Luo N, Wang B, Alvarado JA, Hu Y, Sun Y (2017) Loss of OCRL increases ciliary PI(4,5)P2 in oculocerebrorenal syndrome of Lowe. J Cell Sci. https://doi.org/10.1242/jcs.200857
Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, Schurmans S, Dallapiccola B, Majerus PW, Valente EM, Gleeson JG (2009) Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41(9):1032–1036. https://doi.org/10.1038/ng.423
Chavez M, Ena S, Van Sande J, de Kerchove dA, Schurmans S, Schiffmann SN (2015) Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Dev Cell 34(3):338–350. https://doi.org/10.1016/j.devcel.2015.06.016
Carroll K, Gomez C, Shapiro L (2004) Tubby proteins: the plot thickens. Nat Rev Mol Cell Biol 5(1):55–63. https://doi.org/10.1038/nrm1278
Mehta ZB, Pietka G, Lowe M (2014) The cellular and physiological functions of the Lowe syndrome protein OCRL1. Traffic 15(5):471–487. https://doi.org/10.1111/tra.12160
Boggon TJ, Shan WS, Santagata S, Myers SC, Shapiro L (1999) Implication of tubby proteins as transcription factors by structure-based functional analysis. Science 286(5447):2119–2125
Quinn KV, Behe P, Tinker A (2008) Monitoring changes in membrane phosphatidylinositol 4,5-bisphosphate in living cells using a domain from the transcription factor tubby. J Physiol 586(12):2855–2871. https://doi.org/10.1113/jphysiol.2008.153791
Kim S, Sung HJ, Lee JW, Kim YH, Oh YS, Yoon KA, Heo K, Suh PG (2017) C-terminally mutated tubby protein accumulates in aggresomes. BMB Rep 50(1):37–42
Caberoy NB, Zhou Y, Li W (2010) Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J 29(23):3898–3910. https://doi.org/10.1038/emboj.2010.265
Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N (2003) Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 425(6958):628–633. https://doi.org/10.1038/nature02030
Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, Braun T, Casavant T, Stone EM, Sheffield VC (2004) Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci USA 101(23):8664–8669. https://doi.org/10.1073/pnas.0402354101
Mykytyn K, Sheffield VC (2004) Establishing a connection between cilia and Bardet-Biedl syndrome. Trends Mol Med 10(3):106–109
Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K (2008) Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA 105(11):4242–4246. https://doi.org/10.1073/pnas.0711027105
Zhang Q, Nishimura D, Vogel T, Shao J, Swiderski R, Yin T, Searby C, Carter CS, Kim G, Bugge K, Stone EM, Sheffield VC (2013) BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J Cell Sci 126(Pt 11):2372–2380. https://doi.org/10.1242/jcs.111740
Novas R, Cardenas-Rodriguez M, Irigoin F, Badano JL (2015) Bardet-Biedl syndrome: is it only cilia dysfunction? FEBS Lett 589(22):3479–3491. https://doi.org/10.1016/j.febslet.2015.07.031
Yen HJ, Tayeh MK, Mullins RF, Stone EM, Sheffield VC, Slusarski DC (2006) Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer’s vesicle cilia function. Hum Mol Genet 15(5):667–677. https://doi.org/10.1093/hmg/ddi468
Kudryashova E, Kudryashov D, Kramerova I, Spencer MJ (2005) Trim32 is a ubiquitin ligase mutated in limb girdle muscular dystrophy type 2H that binds to skeletal muscle myosin and ubiquitinates actin. J Mol Biol 354(2):413–424. https://doi.org/10.1016/j.jmb.2005.09.068
Hernandez-Hernandez V, Pravincumar P, Diaz-Font A, May-Simera H, Jenkins D, Knight M, Beales PL (2013) Bardet-Biedl syndrome proteins control the cilia length through regulation of actin polymerization. Hum Mol Genet 22(19):3858–3868. https://doi.org/10.1093/hmg/ddt241
Ridley AJ (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16(10):522–529. https://doi.org/10.1016/j.tcb.2006.08.006
Prevo B, Scholey JM, Peterman EJ (2017) Intraflagellar transport: mechanisms of motor action, cooperation and cargo delivery. FEBS J. https://doi.org/10.1111/febs.14068
Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT (2009) Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol 11(11):1332–1339. https://doi.org/10.1038/ncb1977
Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG (2005) Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem 280(30):27688–27696. https://doi.org/10.1074/jbc.M505062200
Kubo T, Brown JM, Bellve K, Craige B, Craft JM, Fogarty K, Lechtreck KF, Witman GB (2016) Together, the IFT81 and IFT74 N-termini form the main module for intraflagellar transport of tubulin. J Cell Sci 129(10):2106–2119. https://doi.org/10.1242/jcs.187120
Bizet AA, Becker-Heck A, Ryan R, Weber K, Filhol E, Krug P, Halbritter J, Delous M, Lasbennes MC, Linghu B, Oakeley EJ, Zarhrate M, Nitschke P, Garfa-Traore M, Serluca F, Yang F, Bouwmeester T, Pinson L, Cassuto E, Dubot P, Elshakhs NA, Sahel JA, Salomon R, Drummond IA, Gubler MC, Antignac C, Chibout S, Szustakowski JD, Hildebrandt F, Lorentzen E, Sailer AW, Benmerah A, Saint-Mezard P, Saunier S (2015) Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization. Nat Commun 6:8666. https://doi.org/10.1038/ncomms9666
Wang Z, Wann AK, Thompson CL, Hassen A, Wang W, Knight MM (2016) IFT88 influences chondrocyte actin organization and biomechanics. Osteoarthr Cartil 24(3):544–554. https://doi.org/10.1016/j.joca.2015.10.003
You N, Liu W, Tang L, Zhong X, Ji R, Zhang N, Wang D, He Y, Dou K, Tao K (2012) Tg737 signaling is required for hypoxia-enhanced invasion and migration of hepatoma cells. J Exp Clin Cancer Res 31:75. https://doi.org/10.1186/1756-9966-31-75
Boehlke C, Janusch H, Hamann C, Powelske C, Mergen M, Herbst H, Kotsis F, Nitschke R, Kuehn EW (2015) A cilia independent role of Ift88/polaris during cell migration. PLoS One 10(10):e0140378. https://doi.org/10.1371/journal.pone.0140378
Robert A, Margall-Ducos G, Guidotti JE, Bregerie O, Celati C, Brechot C, Desdouets C (2007) The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J Cell Sci 120(Pt 4):628–637. https://doi.org/10.1242/jcs.03366
Pedersen LB, Mogensen JB, Christensen ST (2016) Endocytic control of cellular signaling at the primary cilium. Trends Biochem Sci 41(9):784–797. https://doi.org/10.1016/j.tibs.2016.06.002
Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K (1993) Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol 123(1):35–45
Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129(6):1201–1213. https://doi.org/10.1016/j.cell.2007.03.053
Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W (2010) Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA 107(14):6346–6351. https://doi.org/10.1073/pnas.1002401107
Fu W, Wang L, Kim S, Li J, Dynlacht BD (2016) Role for the IFT-A complex in selective transport to the primary cilium. Cell Rep 17(6):1505–1517. https://doi.org/10.1016/j.celrep.2016.10.018
Peranen J (2011) Rab8 GTPase as a regulator of cell shape. Cytoskeleton (Hoboken) 68(10):527–539. https://doi.org/10.1002/cm.20529
Barral DC, Garg S, Casalou C, Watts GF, Sandoval JL, Ramalho JS, Hsu VW, Brenner MB (2012) Arl13b regulates endocytic recycling traffic. Proc Natl Acad Sci USA 109(52):21354–21359. https://doi.org/10.1073/pnas.1218272110
Casalou C, Seixas C, Portelinha A, Pintado P, Barros M, Ramalho JS, Lopes SS, Barral DC (2014) Arl13b and the non-muscle myosin heavy chain IIA are required for circular dorsal ruffle formation and cell migration. J Cell Sci 127(Pt 12):2709–2722. https://doi.org/10.1242/jcs.143446
Poole CA, Flint MH, Beaumont BW (1985) Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil 5(3):175–193
Poole CA, Jensen CG, Snyder JA, Gray CG, Hermanutz VL, Wheatley DN (1997) Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol Int 21(8):483–494. https://doi.org/10.1006/cbir.1997.0177
Tenkova T, Chaldakov GN (1988) Golgi-cilium complex in rabbit ciliary process cells. Cell Struct Funct 13(5):455–458
Follit JA, Tuft RA, Fogarty KE, Pazour GJ (2006) The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17(9):3781–3792. https://doi.org/10.1091/mbc.E06-02-0133
Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ (2008) The golgin GMAP210/TRIP11 anchors IFT20 to the golgi complex. PLoS Genet 4(12):e1000315. https://doi.org/10.1371/journal.pgen.1000315
Munro S (2011) The golgin coiled–coil proteins of the golgi apparatus. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a005256
Greer YE, Westlake CJ, Gao B, Bharti K, Shiba Y, Xavier CP, Pazour GJ, Yang Y, Rubin JS (2014) Casein kinase 1delta functions at the centrosome and Golgi to promote ciliogenesis. Mol Biol Cell 25(10):1629–1640. https://doi.org/10.1091/mbc.E13-10-0598
Zahnleiter D, Hauer NN, Kessler K, Uebe S, Sugano Y, Neuhauss SC, Giessl A, Ekici AB, Blessing H, Sticht H, Dorr HG, Reis A, Thiel CT (2015) MAP4-dependent regulation of microtubule formation affects centrosome, cilia, and Golgi architecture as a central mechanism in growth regulation. Hum Mutat 36(1):87–97. https://doi.org/10.1002/humu.22711
Goncalves J, Nolasco S, Nascimento R, Lopez Fanarraga M, Zabala JC, Soares H (2010) TBCCD1, a new centrosomal protein, is required for centrosome and Golgi apparatus positioning. EMBO Rep 11(3):194–200. https://doi.org/10.1038/embor.2010.5
Dafinger C, Liebau MC, Elsayed SM, Hellenbroich Y, Boltshauser E, Korenke GC, Fabretti F, Janecke AR, Ebermann I, Nurnberg G, Nurnberg P, Zentgraf H, Koerber F, Addicks K, Elsobky E, Benzing T, Schermer B, Bolz HJ (2011) Mutations in KIF7 link Joubert syndrome with Sonic Hedgehog signaling and microtubule dynamics. J Clin Invest 121(7):2662–2667. https://doi.org/10.1172/JCI43639
Zhang L, Li W, Ni J, Wu J, Liu J, Zhang Z, Zhang Y, Li H, Shi Y, Teves ME, Song S, Strauss JF 3rd, Zhang Z (2015) RC/BTB2 is essential for formation of primary cilia in mammalian cells. Cytoskeleton (Hoboken) 72(4):171–181. https://doi.org/10.1002/cm.21214
Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME (2010) The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet 19(7):1358–1367. https://doi.org/10.1093/hmg/ddq012
Baron Gaillard CL, Pallesi-Pocachard E, Massey-Harroche D, Richard F, Arsanto JP, Chauvin JP, Lecine P, Kramer H, Borg JP, Le Bivic A (2011) Hook2 is involved in the morphogenesis of the primary cilium. Mol Biol Cell 22(23):4549–4562. https://doi.org/10.1091/mbc.E11-05-0405
Joo K, Kim CG, Lee MS, Moon HY, Lee SH, Kim MJ, Kweon HS, Park WY, Kim CH, Gleeson JG, Kim J (2013) CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc Natl Acad Sci USA 110(15):5987–5992. https://doi.org/10.1073/pnas.1220927110
Stoetzel C, Bar S, De Craene JO, Scheidecker S, Etard C, Chicher J, Reck JR, Perrault I, Geoffroy V, Chennen K, Strahle U, Hammann P, Friant S, Dollfus H (2016) A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi. Nat Commun 7:13586. https://doi.org/10.1038/ncomms13586
Hua K, Ferland RJ (2017) Fixation methods can differentially affect ciliary protein immunolabeling. Cilia 6:5. https://doi.org/10.1186/s13630-017-0045-9
Dustin ML, Baldari CT (2017) The immune synapse: past, present, and future. Methods Mol Biol 1584:1–5. https://doi.org/10.1007/978-1-4939-6881-7_1
Finetti F, Onnis A, Baldari CT (2015) Regulation of vesicular traffic at the T cell immune synapse: lessons from the primary cilium. Traffic 16(3):241–249. https://doi.org/10.1111/tra.12241
Geiger B, Rosen D, Berke G (1982) Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol 95(1):137–143
Kupfer A, Dennert G, Singer SJ (1983) Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci USA 80(23):7224–7228
Kupfer A, Dennert G (1984) Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol 133(5):2762–2766
Stinchcombe JC, Randzavola LO, Angus KL, Mantell JM, Verkade P, Griffiths GM (2015) Mother centriole distal appendages mediate centrosome docking at the immunological synapse and reveal mechanistic parallels with ciliogenesis. Curr Biol 25(24):3239–3244. https://doi.org/10.1016/j.cub.2015.10.028
Finetti F, Paccani SR, Rosenbaum J, Baldari CT (2011) Intraflagellar transport: a new player at the immune synapse. Trends Immunol 32(4):139–145. https://doi.org/10.1016/j.it.2011.02.001
Finetti F, Baldari CT (2013) Compartmentalization of signaling by vesicular trafficking: a shared building design for the immune synapse and the primary cilium. Immunol Rev 251(1):97–112. https://doi.org/10.1111/imr.12018
Onnis A, Finetti F, Patrussi L, Gottardo M, Cassioli C, Spano S, Baldari CT (2015) The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death Differ 22(10):1687–1699. https://doi.org/10.1038/cdd.2015.17
Patrussi L, Baldari CT (2016) The Rab GTPase Rab8 as a shared regulator of ciliogenesis and immune synapse assembly: from a conserved pathway to diverse cellular structures. Small GTPases 7(1):16–20. https://doi.org/10.1080/21541248.2015.1111852
Nagano M, Shinoda K (1994) Coexistence of the stigmoid body and estrogen receptor in some neuronal groups involved in rat reproductive functions. Brain Res 634(2):296–304
Shinoda K (1994) Sex-steroid receptor mechanism related to neuronal aromatase and the stigmoid body. Horm Behav 28(4):545–555. https://doi.org/10.1006/hbeh.1994.1053
Li SH, Gutekunst CA, Hersch SM, Li XJ (1998) Association of HAP1 isoforms with a unique cytoplasmic structure. J Neurochem 71(5):2178–2185
Muneoka KT, Takigawa M (2003) 5-Hydroxytryptamine7 (5-HT7) receptor immunoreactivity-positive ‘stigmoid body’-like structure in developing rat brains. Int J Dev Neurosci 21(3):133–143
Takeshita Y, Fujinaga R, Zhao C, Yanai A, Shinoda K (2006) Huntingtin-associated protein 1 (HAP1) interacts with androgen receptor (AR) and suppresses SBMA-mutant-AR-induced apoptosis. Hum Mol Genet 15(15):2298–2312. https://doi.org/10.1093/hmg/ddl156
Ruppersburg CC, Hartzell HC (2014) The Ca2+-activated Cl- channel ANO1/TMEM16A regulates primary ciliogenesis. Mol Biol Cell 25(11):1793–1807. https://doi.org/10.1091/mbc.E13-10-0599
Ramer MS, Cruz Cabrera MA, Alan N, Scott AL, Inskip JA (2010) A new organellar complex in rat sympathetic neurons. PLoS One 5(5):e10872. https://doi.org/10.1371/journal.pone.0010872
Rachel RA, Yamamoto EA, Dewanjee MK, May-Simera HL, Sergeev YV, Hackett AN, Pohida K, Munasinghe J, Gotoh N, Wickstead B, Fariss RN, Dong L, Li T, Swaroop A (2015) CEP290 alleles in mice disrupt tissue-specific cilia biogenesis and recapitulate features of syndromic ciliopathies. Hum Mol Genet 24(13):3775–3791. https://doi.org/10.1093/hmg/ddv123
Hong DH, Pawlyk B, Sokolov M, Strissel KJ, Yang J, Tulloch B, Wright AF, Arshavsky VY, Li T (2003) RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci 44(6):2413–2421
Moore A, Escudier E, Roger G, Tamalet A, Pelosse B, Marlin S, Clement A, Geremek M, Delaisi B, Bridoux AM, Coste A, Witt M, Duriez B, Amselem S (2006) RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet 43(4):326–333. https://doi.org/10.1136/jmg.2005.034868
Bukowy-Bieryllo Z, Zietkiewicz E, Loges NT, Wittmer M, Geremek M, Olbrich H, Fliegauf M, Voelkel K, Rutkiewicz E, Rutland J, Morgan L, Pogorzelski A, Martin J, Haan E, Berger W, Omran H, Witt M (2013) RPGR mutations might cause reduced orientation of respiratory cilia. Pediatr Pulmonol 48(4):352–363. https://doi.org/10.1002/ppul.22632
Shah AS, Farmen SL, Moninger TO, Businga TR, Andrews MP, Bugge K, Searby CC, Nishimura D, Brogden KA, Kline JN, Sheffield VC, Welsh MJ (2008) Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia. Proc Natl Acad Sci USA 105(9):3380–3385. https://doi.org/10.1073/pnas.0712327105
Shoemark A, Dixon M, Beales PL, Hogg CL (2015) Bardet Biedl syndrome: motile ciliary phenotype. Chest 147(3):764–770. https://doi.org/10.1378/chest.13-2913
Miyoshi K, Kasahara K, Murakami S, Takeshima M, Kumamoto N, Sato A, Miyazaki I, Matsuzaki S, Sasaoka T, Katayama T, Asanuma M (2014) Lack of dopaminergic inputs elongates the primary cilia of striatal neurons. PLoS One 9(5):e97918. https://doi.org/10.1371/journal.pone.0097918
Lim YC, McGlashan SR, Cooling MT, Long DS (2015) Culture and detection of primary cilia in endothelial cell models. Cilia 4:11. https://doi.org/10.1186/s13630-015-0020-2
Wang J, Barr MM (2016) Ciliary extracellular vesicles: txt msg organelles. Cell Mol Neurobiol 36(3):449–457. https://doi.org/10.1007/s10571-016-0345-4
Acknowledgements
We thank Julia Nalwalk for helpful discussions and editing of our manuscript. This work was supported in part by an NIH/NINDS R01NS092062 Grant to RJF.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hua, K., Ferland, R.J. Primary cilia proteins: ciliary and extraciliary sites and functions. Cell. Mol. Life Sci. 75, 1521–1540 (2018). https://doi.org/10.1007/s00018-017-2740-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2740-5