Abstract
The retinoic acid (RA) signaling pathway regulates axial patterning and neurogenesis in the developing central nervous system (CNS) of chordates, but little is known about its roles during peripheral nervous system (PNS) formation and about how these roles might have evolved. This study assesses the requirement of RA signaling for establishing a functional PNS in the cephalochordate amphioxus, the best available stand-in for the ancestral chordate condition. Pharmacological manipulation of RA signaling levels during embryogenesis reduces the ability of amphioxus larvae to respond to sensory stimulation and alters the number and distribution of ectodermal sensory neurons (ESNs) in a stage- and context-dependent manner. Using gene expression assays combined with immunohistochemistry, we show that this is because RA signaling specifically acts on a small population of soxb1c-expressing ESN progenitors, which form a neurogenic niche in the trunk ectoderm, to modulate ESN production during elongation of the larval body. Our findings reveal an important role for RA signaling in regulating neurogenic niche activity in the larval amphioxus PNS. Although only few studies have addressed this issue so far, comparable RA signaling functions have been reported for neurogenic niches in the CNS and in certain neurogenic placode derivatives of vertebrates. Accordingly, the here-described mechanism is likely a conserved feature of chordate embryonic and adult neural development.












Similar content being viewed by others
References
Wang Y, Chen J, Du C et al (2014) Characterization of retinoic acid-induced neurobehavioral effects in developing zebrafish. Environ Toxicol Chem 33:431–437. https://doi.org/10.1002/etc.2453
Bailey JM, Oliveri AN, Karbhari N et al (2016) Persistent behavioral effects following early life exposure to retinoic acid or valproic acid in zebrafish. Neurotoxicology 52:23–33. https://doi.org/10.1016/j.neuro.2015.10.001
Carta M, Stancampiano R, Tronci E et al (2006) Vitamin A deficiency induces motor impairments and striatal cholinergic dysfunction in rats. Neuroscience 139:1163–1172. https://doi.org/10.1016/j.neuroscience.2006.01.027
Romand R, Krezel W, Beraneck M et al (2013) Retinoic acid deficiency impairs the vestibular function. J Neurosci 33:5856–5866. https://doi.org/10.1523/JNEUROSCI.4618-12.2013
Srour M, Caron V, Pearson T et al (2016) Gain-of-function mutations in RARB cause intellectual disability with progressive motor impairment. Hum Mutat 37:786–793. https://doi.org/10.1002/humu.23004
Matt N, Dupé V, Garnier JM et al (2005) Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132:4789–4800. https://doi.org/10.1242/dev.02031
Matt N, Ghyselinck NB, Pellerin I, Dupé V (2008) Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol 320:140–148. https://doi.org/10.1016/j.ydbio.2008.04.039
Kumar S, Duester G (2010) Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev Biol 340:67–74. https://doi.org/10.1016/j.ydbio.2010.01.027
Bohnsack BL, Kasprick DS, Kish PE et al (2012) A zebrafish model of Axenfeld–Rieger syndrome reveals that pitx2 regulation by retinoic acid is essential for ocular and craniofacial development. Investig Ophthalmol Vis Sci 53:7–22. https://doi.org/10.1167/iovs.11-8494
Bhasin N, Maynard TM, Gallagher PA, LaMantia AS (2003) Mesenchymal/epithelial regulation of retinoic acid signaling in the olfactory placode. Dev Biol 261:82–98. https://doi.org/10.1016/S0012-1606(03)00295-1
Paschaki M, Cammas L, Muta Y et al (2013) Retinoic acid regulates olfactory progenitor cell fate and differentiation. Neural Dev 8:13. https://doi.org/10.1186/1749-8104-8-13
Romand R, Dollé P, Hashino E (2006) Retinoid signaling in inner ear development. J Neurobiol 66:687–704. https://doi.org/10.1002/neu.20244
Hans S, Christison J, Liu D, Westerfield M (2007) Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol 7:5. https://doi.org/10.1186/1471-213X-7-5
Thiede BR, Mann ZF, Chang W et al (2014) Retinoic acid signalling regulates the development of tonotopically patterned hair cells in the chicken cochlea. Nat Commun 5:3840. https://doi.org/10.1038/ncomms4840
Simkin JE, Zhang D, Rollo BN, Newgreen DF (2013) Retinoic acid upregulates Ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS One 8:e64077. https://doi.org/10.1371/journal.pone.0064077
Laudet V, Zieger E, Schubert M (2015) Evolution of the retinoic acid signaling pathway. In: Dollé P, Neiderreither K (eds) The retinoids. Wiley, Hoboken, pp 75–90
Zieger E, Schubert M (2017) New insights into the roles of retinoic acid signaling in nervous system development and the establishment of neurotransmitter systems. Int Rev Cell Mol Biol 330:1–84. https://doi.org/10.1016/bs.ircmb.2016.09.001
Chambon P (1996) A decade of molecular biology of retinoic acid receptors. FASEB J 10:940–954
Moutier E, Ye T, Choukrallah MA et al (2012) Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J Biol Chem 287:26328–26341. https://doi.org/10.1074/jbc.M112.361790
Vilhais-Neto GC, Pourquié O (2008) Retinoic acid. Curr Biol 18:R191–R192. https://doi.org/10.1016/j.cub.2007.12.042
Gronemeyer H, Gustafsson JA, Laudet V (2004) Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 3:950–964. https://doi.org/10.1038/nrd1551
Rhinn M, Dollé P (2012) Retinoic acid signalling during development. Development 139:843–858. https://doi.org/10.1242/dev.065938
Pennati R, Dell’Anna A, Zega G et al (2013) Retinoic acid influences antero-posterior positioning of peptidergic neurons in the planula larva of the hydrozoan Clava multicornis. Mar Ecol 34:143–152. https://doi.org/10.1111/maec.12032
Farrar NR, Dmetrichuk JM, Carlone RL, Spencer GE (2009) A novel, nongenomic mechanism underlies retinoic acid-induced growth cone turning. J Neurosci 29:14136–14142. https://doi.org/10.1523/JNEUROSCI.2921-09.2009
Carter CJ, Farrar N, Carlone RL, Spencer GE (2010) Developmental expression of a molluscan RXR and evidence for its novel, nongenomic role in growth cone guidance. Dev Biol 343:124–137. https://doi.org/10.1016/j.ydbio.2010.03.023
Carter CJ, Rand C, Mohammad I et al (2015) Expression of a retinoic acid receptor (RAR)-like protein in the embryonic and adult nervous system of a protostome species. J Exp Zool B Mol Dev Evol 324:51–67. https://doi.org/10.1002/jez.b.22604
Sasakura Y, Kanda M, Ikeda T et al (2012) Retinoic acid-driven Hox1 is required in the epidermis for forming the otic/atrial placodes during ascidian metamorphosis. Development 139:2156–2160. https://doi.org/10.1242/dev.080234
Pasini A, Manenti R, Rothbächer U, Lemaire P (2012) Antagonizing retinoic acid and FGF/MAPK pathways control posterior body patterning in the invertebrate chordate Ciona intestinalis. PLoS One 7:e46193. https://doi.org/10.1371/journal.pone.0046193
Schubert M, Holland ND, Escriva H et al (2004) Retinoic acid influences anteroposterior positioning of epidermal sensory neurons and their gene expression in a developing chordate (amphioxus). Proc Natl Acad Sci USA 101:10320–10325. https://doi.org/10.1073/pnas.0403216101
Schubert M, Escriva H, Xavier-Neto J, Laudet V (2006) Amphioxus and tunicates as evolutionary model systems. Trends Ecol Evol 21:269–277. https://doi.org/10.1016/j.tree.2006.01.009
Onai T, Lin HC, Schubert M et al (2009) Retinoic acid and Wnt/β-catenin have complementary roles in anterior/posterior patterning embryos of the basal chordate amphioxus. Dev Biol 332:223–233. https://doi.org/10.1016/j.ydbio.2009.05.571
Zieger E, Candiani S, Garbarino G et al (2018) Roles of retinoic acid signaling in shaping the neuronal architecture of the developing amphioxus nervous system. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0727-8
Delsuc F, Brinkmann H, Chourrout D, Philippe H (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439:965–968. https://doi.org/10.1038/nature04336
Bertrand S, Escriva H (2011) Evolutionary crossroads in developmental biology: amphioxus. Development 138:4819–4830. https://doi.org/10.1242/dev.066720
Yue JX, Yu JK, Putnam NH, Holland LZ (2014) The transcriptome of an amphioxus, Asymmetron lucayanum, from the Bahamas: a window into chordate evolution. Genome Biol Evol 6:2681–2696. https://doi.org/10.1093/gbe/evu212
Holland LZ (2015) Evolution of basal deuterostome nervous systems. J Exp Biol 218:637–645. https://doi.org/10.1242/jeb.109108
Bourlat SJ, Juliusdottir T, Lowe CJ et al (2006) Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature 444:85–88. https://doi.org/10.1038/nature05241
Putnam NH, Butts T, Ferrier DE et al (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453:1064–1071. https://doi.org/10.1038/nature06967
Holland LZ (2013) Evolution of new characters after whole genome duplications: insights from amphioxus. Semin Cell Dev Biol 24:101–109. https://doi.org/10.1016/j.semcdb.2012.12.007
Holland LZ (2015) Genomics, evolution and development of amphioxus and tunicates: the Goldilocks principle. J Exp Zool B Mol Dev Evol 324:342–352. https://doi.org/10.1002/jez.b.22569
Escriva H, Holland ND, Gronemeyer H et al (2002) The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development 129:2905–2916
Schubert M, Holland ND, Laudet V, Holland LZ (2006) A retinoic acid-Hox hierarchy controls both anterior/posterior patterning and neuronal specification in the developing central nervous system of the cephalochordate amphioxus. Dev Biol 296:190–202. https://doi.org/10.1016/j.ydbio.2006.04.457
Koop D, Holland ND, Sémon M et al (2010) Retinoic acid signaling targets Hox genes during the amphioxus gastrula stage: insights into early anterior–posterior patterning of the chordate body plan. Dev Biol 338:98–106. https://doi.org/10.1016/j.ydbio.2009.11.016
Albuixech-Crespo B, López-Blanch L, Burguera D et al (2017) Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLoS Biol 15:e2001573. https://doi.org/10.1371/journal.pbio.2001573
Wicht H, Lacalli TC (2005) The nervous system of amphioxus: structure, development, and evolutionary significance. Can J Zool 83:122–150
Zieger E, Lacalli TC, Pestarino M et al (2017) The origin of dopaminergic systems in chordate brains: insights from amphioxus. Int J Dev Biol 61:749–761. https://doi.org/10.1387/ijdb.170153sc
Holland LZ (2015) The origin and evolution of chordate nervous systems. Philos Trans R Soc B Biol Sci 370:20150048. https://doi.org/10.1098/rstb.2015.0048
Le Petillon Y, Luxardi G, Scerbo P et al (2017) Nodal/Activin pathway is a conserved neural induction signal in chordates. Nat Ecol Evol 1:1192–1200. https://doi.org/10.1038/s41559-017-0226-3
Kaltenbach SL, Yu JK, Holland ND (2009) The origin and migration of the earliest-developing sensory neurons in the peripheral nervous system of amphioxus. Evol Dev 11:142–151. https://doi.org/10.1111/j.1525-142X.2009.00315.x
Lu TM, Luo YJ, Yu JK (2012) BMP and Delta/Notch signaling control the development of amphioxus epidermal sensory neurons: insights into the evolution of the peripheral sensory system. Development 139:2020–2030. https://doi.org/10.1242/dev.073833
Meulemans D, Bronner-Fraser M (2007) The amphioxus SoxB family: implications for the evolution of vertebrate placodes. Int J Biol Sci 3:356–364. https://doi.org/10.7150/ijbs.3.356
Satoh G, Wang Y, Zhang P, Satoh N (2001) Early development of amphioxus nervous system with special reference to segmental cell organization and putative sensory cell precursors: a study based on the expression of pan-neuronal marker gene Hu/elav. J Exp Zool B Mol Dev Evol 291:354–364. https://doi.org/10.1002/jez.1134
Mazet F, Masood S, Luke GN et al (2004) Expression of AmphiCoe, an amphioxus COE/EBF gene, in the developing central nervous system and epidermal sensory neurons. Genesis 38:58–65. https://doi.org/10.1002/gene.20006
Kozmik Z, Holland ND, Kreslova J et al (2007) Pax–Six–Eya–Dach network during amphioxus development: conservation in vitro but context specificity in vivo. Dev Biol 306:143–159. https://doi.org/10.1016/j.ydbio.2007.03.009
Holland ND, Yu JK (2002) Epidermal receptor development and sensory pathways in vitally stained amphioxus (Branchiostoma floridae). Acta Zool 83:309–319. https://doi.org/10.1046/j.1463-6395.2002.00120.x
Candiani S, Moronti L, Ramoino P et al (2012) A neurochemical map of the developing amphioxus nervous system. BMC Neurosci 13:59. https://doi.org/10.1186/1471-2202-13-59
Fuentes M, Schubert M, Dalfo D et al (2004) Preliminary observations on the spawning conditions of the European amphioxus (Branchiostoma lanceolatum) in captivity. J Exp Zool B Mol Dev Evol 302:384–391. https://doi.org/10.1002/jez.b.20025
Fuentes M, Benito E, Bertrand S et al (2007) Insights into spawning behavior and development of the European amphioxus Branchiostoma lanceolatum. J Exp Zool B Mol Dev Evol 308:484–493. https://doi.org/10.1002/jez.b.21179
Theodosiou M, Colin A, Schulz J et al (2011) Amphioxus spawning behavior in an artificial seawater facility. J Exp Zool B Mol Dev Evol 316:263–275. https://doi.org/10.1002/jez.b.21397
Holland LZ, Yu JK (2004) Cephalochordate (amphioxus) embryos: procurement, culture, and basic methods. Methods Cell Biol 74:195–215. https://doi.org/10.1016/S0091-679X(04)74009-1
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Oulion S, Bertrand S, Belgacem MR et al (2012) Sequencing and analysis of the Mediterranean amphioxus (Branchiostoma lanceolatum) transcriptome. PLoS One 7:e36554. https://doi.org/10.1371/journal.pone.0036554
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. https://doi.org/10.1093/bioinformatics/btp348
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771. https://doi.org/10.1080/10635150802429642
Darriba D, Taboada GL, Doallo R, Posada D (2011) ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27:1164–1165. https://doi.org/10.1093/bioinformatics/btr088
Yu JK, Holland LZ (2009) Extraction of RNA from amphioxus embryos or adult amphioxus tissue. Cold Spring Harb Protoc 2009:pdb.prot5288. https://doi.org/10.1101/pdb.prot5288
Yu JK, Holland LZ (2009) Amphioxus whole-mount in situ hybridization. Cold Spring Harb Protoc 2009:pdb.prot5286. https://doi.org/10.1101/pdb.prot5286
Bayascas JR, Yuste VJ, Benito E et al (2002) Isolation of AmphiCASP-3/7, an ancestral caspase from amphioxus (Branchiostoma floridae). Evolutionary considerations for vertebrate caspases. Cell Death Differ 9:1078–1089. https://doi.org/10.1038/sj.cdd.4401075
Stokes MD (1997) Larval locomotion of the lancelet Branchiostoma floridae. J Exp Biol 200:1661–1680
McEntee WJ, Crook TH (1993) Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology 111:391–401. https://doi.org/10.1007/BF02253527
Benito-Gutiérrez È (2006) A gene catalogue of the amphioxus nervous system. Int J Biol Sci 2:149–160. https://doi.org/10.7150/ijbs.2.149
Lacalli TC (2004) Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain Behav Evol 64:148–162. https://doi.org/10.1159/000079744
Lacalli TC, Gilmour THJ, Kelly SJ (1999) The oral nerve plexus in amphioxus larvae: function, cell types and phylogenetic significance. Proc R Soc B Biol Sci 266:1461–1470. https://doi.org/10.1098/rspb.1999.0801
Benito-Gutiérrez È, Illas M, Comella JX, Garcia-Fernàndez J (2005) Outlining the nascent nervous system of Branchiostoma floridae (amphioxus) by the pan-neural marker AmphiElav. Brain Res Bull 66:518–521. https://doi.org/10.1016/j.brainresbull.2005.03.007
Sobreira TJ, Marletaz F, Simoes-Costa M et al (2011) Structural shifts of aldehyde dehydrogenase enzymes were instrumental for the early evolution of retinoid-dependent axial patterning in metazoans. Proc Natl Acad Sci USA 108:226–231. https://doi.org/10.1073/pnas.1011223108
Carvalho JE, Theodosiou M, Chen J et al (2017) Lineage-specific duplication of amphioxus retinoic acid degrading enzymes (CYP26) resulted in sub-functionalization of patterning and homeostatic roles. BMC Evol Biol 17:24. https://doi.org/10.1186/s12862-016-0863-1
Shimozono S, Iimura T, Kitaguchi T et al (2013) Visualization of an endogenous retinoic acid gradient across embryonic development. Nature 496:363–366. https://doi.org/10.1038/nature12037
Scott SH (2004) Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci 5:532–546. https://doi.org/10.1038/nrn1427
Windhorst U (2007) Muscle proprioceptive feedback and spinal networks. Brain Res Bull 73:155–202. https://doi.org/10.1016/j.brainresbull.2007.03.010
Duester G (2013) Retinoid signaling in control of progenitor cell differentiation during mouse development. Semin Cell Dev Biol 24:694–700. https://doi.org/10.1016/j.semcdb.2013.08.001
Janesick A, Wu SC, Blumberg B (2015) Retinoic acid signaling and neuronal differentiation. Cell Mol Life Sci 72:1559–1576. https://doi.org/10.1007/s00018-014-1815-9
Gudas LJ (2013) Retinoids induce stem cell differentiation via epigenetic changes. Semin Cell Dev Biol 24:701–705. https://doi.org/10.1016/j.semcdb.2013.08.002
Schug TT, Berry DC, Shaw NS et al (2007) Dual transcriptional activities underlie opposing effects of retinoic acid on cell survival. Cell 129:723–733. https://doi.org/10.1016/j.cell.2007.02.050
Wolf G (2008) Retinoic acid as cause of cell proliferation or cell growth inhibition depending on activation of one of two different nuclear receptors. Nutr Rev 66:55–59. https://doi.org/10.1111/j.1753-4887.2007.00006.x
Richards GS, Rentzsch F (2014) Transgenic analysis of a SoxB gene reveals neural progenitor cells in the cnidarian Nematostella vectensis. Development 141:4681–4689. https://doi.org/10.1242/dev.112029
Stollewerk A (2016) A flexible genetic toolkit for arthropod neurogenesis. Philos Trans R Soc B Biol Sci 371:20150044. https://doi.org/10.1098/rstb.2015.0044
Burke RD, Moller DJ, Krupke OA, Taylor VJ (2014) Sea urchin neural development and the metazoan paradigm of neurogenesis. Genesis 52:208–221. https://doi.org/10.1002/dvg.22750
Garner S, Zysk I, Byrne G et al (2016) Neurogenesis in sea urchin embryos and the diversity of deuterostome neurogenic mechanisms. Development 143:286–297. https://doi.org/10.1242/dev.124503
Cunningham D, Casey ES (2014) Spatiotemporal development of the embryonic nervous system of Saccoglossus kowalevskii. Dev Biol 386:252–263. https://doi.org/10.1016/j.ydbio.2013.12.001
Hartenstein V, Stollewerk A (2015) The evolution of early neurogenesis. Dev Cell 32:390–407. https://doi.org/10.1016/j.devcel.2015.02.004
Koop D, Chen J, Theodosiou M et al (2014) Roles of retinoic acid and Tbx1/10 in pharyngeal segmentation: amphioxus and the ancestral chordate condition. EvoDevo 5:36. https://doi.org/10.1186/2041-9139-5-36
Holland LZ (2009) Chordate roots of the vertebrate nervous system: expanding the molecular toolkit. Nat Rev Neurosci 10:736–746. https://doi.org/10.1038/nrn2703
Ohtsuka Y, Matsumoto J, Katsuyama Y, Okamura Y (2014) Nodal signaling regulates specification of ascidian peripheral neurons through control of the BMP signal. Development 141:3889–3899. https://doi.org/10.1242/dev.110213
Waki K, Imai KS, Satou Y (2015) Genetic pathways for differentiation of the peripheral nervous system in ascidians. Nat Commun 6:8719. https://doi.org/10.1038/ncomms9719
Abitua PB, Gainous TB, Kaczmarczyk AN et al (2015) The pre-vertebrate origins of neurogenic placodes. Nature 524:462–465. https://doi.org/10.1038/nature14657
Stolfi A, Ryan K, Meinertzhagen IA, Christiaen L (2015) Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature 527:371–374. https://doi.org/10.1038/nature15758
Cheng L, Arata A, Mizuguchi R et al (2004) Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci 7:510–517. https://doi.org/10.1038/nn1221
Murphy GJ, Glickfeld LL, Balsen Z, Isaacson JS (2004) Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci 24:3023–3030. https://doi.org/10.1523/JNEUROSCI.5745-03.2004
Tucker ES, Lehtinen MK, Maynard T et al (2010) Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development 137:2471–2481. https://doi.org/10.1242/dev.049718
Haskell GT, LaMantia AS (2005) Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci 25:7636–7647. https://doi.org/10.1523/JNEUROSCI.0485-05.2005
Rajaii F, Bitzer ZT, Xu Q, Sockanathan S (2008) Expression of the dominant negative retinoid receptor, RAR403, alters telencephalic progenitor proliferation, survival, and cell fate specification. Dev Biol 316:371–382. https://doi.org/10.1016/j.ydbio.2008.01.041
Wilson L, Gale E, Maden M (2003) The role of retinoic acid in the morphogenesis of the neural tube. J Anat 203:357–368. https://doi.org/10.1046/j.1469-7580.2003.00230.x
England S, Batista MF, Mich JK et al (2011) Roles of Hedgehog pathway components and retinoic acid signalling in specifying zebrafish ventral spinal cord neurons. Development 138:5121–5134. https://doi.org/10.1242/dev.066159
Mich JK, Chen JK (2011) Hedgehog and retinoic acid signaling cooperate to promote motoneurogenesis in zebrafish. Development 138:5113–5119. https://doi.org/10.1242/dev.066225
Sockanathan S, Jessell TM (1998) Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell 94:503–514. https://doi.org/10.1016/S0092-8674(00)81591-3
Janesick A, Shiotsugu J, Taketani M, Blumberg B (2012) RIPPLY3 is a retinoic acid-inducible repressor required for setting the borders of the pre-placodal ectoderm. Development 139:1213–1224. https://doi.org/10.1242/dev.071456
Jaurena MB, Juraver-Geslin H, Devotta A, Saint-Jeannet JP (2015) Zic1 controls placode progenitor formation non-cell autonomously by regulating retinoic acid production and transport. Nat Commun 6:7476. https://doi.org/10.1038/ncomms8476
Maden M, Blentic A, Reijntjes S et al (2007) Retinoic acid is required for specification of the ventral eye field and for Rathke’s pouch in the avian embryo. Int J Dev Biol 51:191–200. https://doi.org/10.1387/ijdb.062175mm
Acknowledgements
The authors would like to thank Thurston C. Lacalli, Nicholas D. Holland, and Linda Z. Holland for fruitful discussions. We are also grateful to Ram Reshef for his vital support with administrative issues.
Funding
This work was supported by a grant from the Agence Nationale de la Recherche (ANR-11-JSV2-002-01) and by funds from the Réseau André Picard (ANR-11-IDEX-0004-02, Sorbonne Universities) to MS and by a National Grant of the University of Genoa (2015) to SC. EZ was a doctoral fellow of the Studienstiftung der Deutschen Wirtschaft (SDW).
Author information
Authors and Affiliations
Contributions
EZ designed and performed experiments, analyzed and interpreted data, and wrote the manuscript. GG supported the collection of gene expression data, NSMR carried out phylogenetic analyses, and JKY contributed important advice concerning the selection of candidate genes. JKY, JCC, and SC provided methodological assistance, supported data analyses, and commented the manuscript. MS designed and supervised the study, analyzed and interpreted data, and wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data used in this study are included in this published article and its supplementary materials.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1: Movie S1. Movie showing reactions of amphioxus larvae at 48 hpf (hours post fertilization) to mechanical stimulation. Response A = quick muscular swimming movement away from the stimulus. Response B = intense wiggling and bending movements without clear directionality. Response C = short wiggling motion on the spot. Response D = short disconnected twitches or bends on the spot. Response E = no visible reaction (MP4 26973 kb)
Additional file 2: Movie S2. Movie showing responses of amphioxus larvae at 48 hpf (hours post fertilization) to chemical stimulation. As indicated in the movie, the amphioxus embryos were exposed to dimethyl sulfoxide (DMSO) (Control), the retinoic acid receptor (RAR) antagonist BMS493 or all-trans retinoic acid (RA), starting from treatment time points (t) at 6 or 24 hpf. Upon reaching the 48 hpf stage, the larvae were further exposed to agarose blocks, which had either been dissolved in artificial seawater (negative control) or in artificial seawater supplemented with 0.1 M l-glutamate (MP4 88999 kb)
Additional file 3: Figure S1.
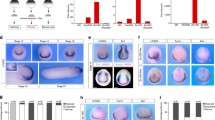
Effects of retinoic acid (RA) signaling alterations on glutamate chemoreception and larval circling/spiraling behavior in amphioxus. Amphioxus embryos were exposed to dimethyl sulfoxide (DMSO) (Control, green bars), the RA receptor (RAR) antagonist BMS493 (blue bars) or all-trans RA (red bars), starting from treatment time points (t) at 6 or 24 hpf (hours post fertilization). Subsequently, by 48 hpf, the animals were further exposed to agarose blocks, which had either been dissolved in artificial seawater (negative control, dark-colored bars) or in artificial seawater supplemented with 0.1 M l-glutamate (light-colored bars). The size of the colored bars indicates the average number of circles amphioxus larvae swam without interruption and the error bars indicate the standard deviation (σ). The total number (n) of animals counted is given at the base of each colored bar. Asterisks (*) above an error bar indicate that the difference between this condition and the corresponding control is statistically significant with a p-value < 0.05 (one asterisk, *) or with a p-value < 0.01 (two asterisks, **). Only larvae that had passed by the agarose block within a 1 cm radius and within 0.5 to 10 min after introduction of the agarose block were taken into consideration. For RA treatments at 6 hpf, circling/spiraling was rarely observed, precluding statistical analyses (PNG 89 kb)
Additional file 4: Table S1.
GenBank accession numbers of sequences used for phylogenetic analyses (DOCX 38 kb)
Additional file 5: Figure S2.
Maximum-likelihood phylogenies for a ELAV, b TLX, and c SOXB1. Trees were inferred using RAxML with 1000 rapid bootstraps (PDF 198 kb)
Additional file 6: Figure S3.
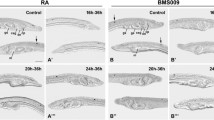
Effects of retinoic acid (RA) signaling alterations on the development of vglut-expressing cells in amphioxus. Larvae are shown in lateral (a-i) or dorsal (d’-i’) view with their anterior ends directed towards the right. Developmental stages are given as hours post fertilization (hpf). Embryos have been treated from the treatment stage (t) at 6 hpf with dimethyl sulfoxide (DMSO) (Control), the RA receptor (RAR) antagonist BMS493 or all-trans RA, as indicated. All scale bars are 50 µm. The scale bar in a applies also to b,c, the scale bar in d applies also to d’,e,e’,f,f’, and the scale bar in g applies also to g’,h,h’,i,i’ (TIFF 1376 kb)
Additional file 7: Figure S4.
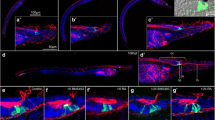
Expression of hu/elav during amphioxus development. a-e Lateral views of amphioxus embryos and larvae at different developmental stages from 15 to 36 hpf (hours post fertilization). Anterior ends are directed towards the right. a’-e’ Dorsal views of the amphioxus embryos and larvae shown, respectively, in a-e. c-e Dotted boxes indicate the ectodermal domain containing a conspicuously high density of hu/elav-expressing ectodermal sensory neuron progenitors (ESNPs). All scale bars are 50 µm. The scale bar in a, b, c, d, and e, respectively, also applies to a’, b’, c’, d’, and e’ (TIFF 3994 kb)
Additional file 8: Figure S5.
Expression of tlx during amphioxus development. a-e Lateral views of amphioxus embryos and larvae at different developmental stages from 15 to 36 hpf (hours post fertilization). Anterior ends are directed towards the right. a’-e’ Dorsal views of the amphioxus embryos and larvae shown, respectively, in a-e. c-e Dotted boxes indicate the ectodermal domain containing a conspicuously high density of ectodermal sensory neurons (ESNs) (compare with Fig. 3), hu/elav-expressing ESN progenitors (ESNPs) (compare with Additional file 7: Figure S4) as well as soxb1c-expressing ESNPs (compare with Additional file 9: Figure S6), but not tlx-expressing ESNPs. All scale bars are 50 µm. The scale bar in a, b, c, d, and e, respectively, also applies to a’, b’, c’, d’, and e’ (TIFF 3531 kb)
Additional file 9: Figure S6.
Expression of soxb1c during amphioxus development. a-e Lateral views of amphioxus embryos and larvae at different developmental stages from 15 to 36 hpf (hours post fertilization). The anterior is directed towards the right. a’-e’ Dorsal views of the amphioxus embryos and larvae shown, respectively, in a-e. The images in c-e’ are focused on soxb1c expression in the ectoderm. Dotted boxes indicate the ectodermal domain containing a conspicuously high density of soxb1c-expressing ectodermal sensory neuron progenitors (ESNPs). All scale bars are 50 µm. The scale bar in a, b, c, d, and e, respectively, also applies to a’, b’, c’, d’, and e’ (TIFF 3617 kb)
Rights and permissions
About this article
Cite this article
Zieger, E., Garbarino, G., Robert, N.S.M. et al. Retinoic acid signaling and neurogenic niche regulation in the developing peripheral nervous system of the cephalochordate amphioxus. Cell. Mol. Life Sci. 75, 2407–2429 (2018). https://doi.org/10.1007/s00018-017-2734-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2734-3