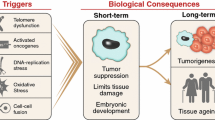
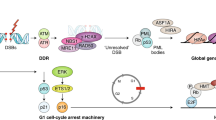
Abstract
Senescence is a cell state occurring in vitro and in vivo after successive replication cycles and/or upon exposition to various stressors. It is characterized by a strong cell cycle arrest associated with several molecular, metabolic and morphologic changes. The accumulation of senescent cells in tissues and organs with time plays a role in organismal aging and in several age-associated disorders and pathologies. Moreover, several therapeutic interventions are able to prematurely induce senescence. It is, therefore, tremendously important to characterize in-depth, the mechanisms by which senescence is induced, as well as the precise properties of senescent cells. For historical reasons, senescence is often studied with fibroblast models. Other cell types, however, much more relevant regarding the structure and function of vital organs and/or regarding pathologies, are regrettably often neglected. In this article, we will clarify what is known on senescence of epithelial cells and highlight what distinguishes it from, and what makes it like, replicative senescence of fibroblasts taken as a standard.



Similar content being viewed by others
Abbreviations
- BER:
-
Base excision repair
- BPEC:
-
Breast primary epithelial cell
- CKI:
-
Cyclin-dependent kinase inhibitor
- DDR:
-
DNA damage response
- DSB:
-
Double-strand break
- EMT:
-
Epithelium-to-mesenchyme transition
- HMEC:
-
Human mammary epithelial cell
- NHEK:
-
Normal human epidermal keratinocyte
- NHOK:
-
Normal human oral keratinocyte
- OIS:
-
Oncogene-induced senescence
- PARP1:
-
Poly(ADP)ribose polymerase 1
- PD:
-
Population doubling
- PSNE:
-
Post-senescence neoplastic emergence
- ROS:
-
Reactive oxygen species
- RS:
-
Replicative senescence
- SA-β-Gal:
-
Senescence-associated-β-galactosidase activity
- SAHF:
-
Senescence-associated heterochromatin foci
- SASP:
-
Senescence-associated secretory phenotype
- SIPS:
-
Stress-induced premature senescence
- SMS:
-
Senescence messaging secretome
- SSB:
-
Single-strand break
- SSBR:
-
Single-strand break repair
- TIS:
-
Therapy-induced senescence
- UPR:
-
Unfolded protein response
References
Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37:614–636
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Vaziri H, Benchimol S (1996) From telomere loss to p53 induction and activation of a DNA-damage pathway at senescence: the telomere loss/DNA damage model of cell aging. Exp Gerontol 31:295–301
Hezel AF, Bardeesy N, Maser RS (2005) Telomere induced senescence: end game signaling. Curr Mol Med 5:145–152
Deng Y, Chan SS, Chang S (2008) Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8:450–458
Criscione SW, Teo YV, Neretti N (2016) The chromatin landscape of cellular senescence. Trends Genet 32:751–761
Kuwano K, Araya J, Hara H, Minagawa S, Takasaka N, Ito S, Kobayashi K, Nakayama K (2016) Cellular senescence and autophagy in the pathogenesis of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). Respir Investig 54:397–406
Parry AJ, Narita M (2016) Old cells, new tricks: chromatin structure in senescence. Mamm Genome 27:320–331
Salama R, Sadaie M, Hoare M, Narita M (2014) Cellular senescence and its effector programs. Genes Dev 28:99–114
Wiley CD, Campisi J (2016) From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab 23:1013–1021
Urbanelli L, Buratta S, Sagini K, Tancini B, Emiliani C (2016) Extracellular vesicles as new players in cellular senescence. Int J Mol Sci 17:1408
Demaria M, Desprez PY, Campisi J, Velarde MC (2015) Cell autonomous and non-autonomous effects of senescent cells in the skin. J Invest Dermatol 135:1722–1726
Panebianco C, Oben JA, Vinciguerra M, Pazienza V (2016) C. Clin Exp Med
Adnot S, Amsellem V, Boyer L, Marcos E, Saker M, Houssaini A, Kebe K, Dagouassat M, Lipskaia L, Boczkowski J (2015) Telomere dysfunction and cell senescence in chronic lung diseases: therapeutic potential. Pharmacol Ther 153:125–134
Tan FC, Hutchison ER, Eitan E, Mattson MP (2014) Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology 15:643–660
Sharpless NE, Sherr CJ (2015) Forging a signature of in vivo senescence. Nat Rev Cancer 15:397–408
Childs BG, Durik M, Baker DJ, van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21:1424–1435
Foster SA, Galloway DA (1996) Human papillomavirus type 16 E7 alleviates a proliferation block in early passage human mammary epithelial cells. Oncogene 12:1773–1779
Nassour J, Martien S, Martin N, Deruy E, Tomellini E, Malaquin N, Bouali F, Sabatier L, Wernert N, Pinte S, Gilson E, Pourtier A, Pluquet O, Abbadie C (2016) Defective DNA single-strand break repair is responsible for senescence and neoplastic escape of epithelial cells. Nat Commun 7:10399
Gosselin K, Martien S, Pourtier A, Vercamer C, Ostoich P, Morat L, Sabatier L, Duprez L, T’Kint de Roodenbeke C, Gilson E, Malaquin N, Wernert N, Slijepcevic P, Ashtari M, Chelli F, Deruy E, Vandenbunder B, De Launoit Y, Abbadie C (2009) Senescence-associated oxidative DNA damage promotes the generation of neoplastic cells. Cancer Res 69:7917–7925
Stampfer MR, Bartley JC (1985) Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci USA 82:2394–2398
Brenner AJ, Stampfer MR, Aldaz CM (1998) Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 17:199–205
Jang DH, Bhawal UK, Min HK, Kang HK, Abiko Y, Min BM (2015) A transcriptional roadmap to the senescence and differentiation of human oral keratinocytes. J Gerontol A Biol Sci Med Sci 70:20–32
Romanov SR, Kozakiewicz BK, Holst CR, Stampfer MR, Haupt LM, Tlsty TD (2001) Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature 409:633–637
Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricala C, O’Toole KM (2002) A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol 22:5157–5172
Feijoo P, Terradas M, Soler D, Dominguez D, Tusell L, Genesca A (2016) Breast primary epithelial cells that escape p16-dependent stasis enter a telomere-driven crisis state. Breast Cancer Res 18:7
Martin N, Salazar-Cardozo C, Vercamer C, Ott L, Marot G, Slijepcevic P, Abbadie C, Pluquet O (2014) Identification of a gene signature of a pre-transformation process by senescence evasion in normal human epidermal keratinocytes. Mol Cancer 13:151
Malaquin N, Vercamer C, Bouali F, Martien S, Deruy E, Wernert N, Chwastyniak M, Pinet F, Abbadie C, Pourtier A (2013) Senescent fibroblasts enhance early skin carcinogenic events via a paracrine MMP-PAR-1 axis. PLoS One 8:e63607
Gosselin K, Deruy E, Martien S, Vercamer C, Bouali F, Dujardin T, Slomianny C, Houel-Renault L, Chelli F, De Launoit Y, Abbadie C (2009) Senescent keratinocytes die by autophagic programmed cell death. Am J Pathol 174:423–435
Deruy E, Nassour J, Martin N, Vercamer C, Malaquin N, Bertout J, Chelli F, Pourtier A, Pluquet O, Abbadie C (2014) Level of macroautophagy drives senescent keratinocytes into cell death or neoplastic evasion. Cell Death Dis 5:e1577
Nickoloff BJ, Lingen MW, Chang BD, Shen M, Swift M, Curry J, Bacon P, Bodner B, Roninson IB (2004) Tumor suppressor maspin is up-regulated during keratinocyte senescence, exerting a paracrine antiangiogenic activity. Cancer Res 64:2956–2961
Bertram C, Hass R (2008) MMP-7 is involved in the aging of primary human mammary epithelial cells (HMEC). Exp Gerontol 43:209–217
Sandhu C, Donovan J, Bhattacharya N, Stampfer M, Worland P, Slingerland J (2000) Reduction of Cdc25A contributes to cyclin E1-Cdk2 inhibition at senescence in human mammary epithelial cells. Oncogene 19:5314–5323
Norsgaard H, Clark BF, Rattan SI (1996) Distinction between differentiation and senescence and the absence of increased apoptosis in human keratinocytes undergoing cellular aging in vitro. Exp Gerontol 31:563–570
Ince TA, Richardson AL, Bell GW, Saitoh M, Godar S, Karnoub AE, Iglehart JD, Weinberg RA (2007) Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell 12:160–170
Hammond SL, Ham RG, Stampfer MR (1984) Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci USA 81:5435–5439
Stampfer MR, Yaswen P (2000) Culture models of human mammary epithelial cell transformation. J Mammary Gland Biol Neoplasia 5:365–378
Garbe JC, Bhattacharya S, Merchant B, Bassett E, Swisshelm K, Feiler HS, Wyrobek AJ, Stampfer MR (2009) Molecular distinctions between stasis and telomere attrition senescence barriers shown by long-term culture of normal human mammary epithelial cells. Cancer Res 69:7557–7568
Fu B, Quintero J, Baker CC (2003) Keratinocyte growth conditions modulate telomerase expression, senescence, and immortalization by human papillomavirus type 16 E6 and E7 oncogenes. Cancer Res 63:7815–7824
Bekker-Jensen S, Mailand N (2010) Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair (Amst) 9:1219–1228
Rossiello F, Herbig U, Longhese MP, Fumagalli M, d’Adda di Fagagna F (2014) Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr Opin Genet Dev 26:89–95
Vaziri H, Benchimol S (1998) Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol 8:279–282
Brown JP, Wei W, Sedivy JM (1997) Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277:831–834
Lee G, Park BS, Han SE, Oh JE, You YO, Baek JH, Kim GS, Min BM (2000) Concurrence of replicative senescence and elevated expression of p16(INK4A) with subculture-induced but not calcium-induced differentiation in normal human oral keratinocytes. Arch Oral Biol 45:809–818
Foster SA, Wong DJ, Barrett MT, Galloway DA (1998) Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol Cell Biol 18:1793–1801
Garbe J, Wong M, Wigington D, Yaswen P, Stampfer MR (1999) Viral oncogenes accelerate conversion to immortality of cultured conditionally immortal human mammary epithelial cells. Oncogene 18:2169–2180
Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ (1998) Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84–88
Kim RH, Kang MK, Kim T, Yang P, Bae S, Williams DW, Phung S, Shin KH, Hong C, Park NH (2015) Regulation of p53 during senescence in normal human keratinocytes. Aging Cell 14:838–846
Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG (2000) Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 20:1436–1447
Stampfer MR, Bodnar A, Garbe J, Wong M, Pan A, Villeponteau B, Yaswen P (1997) Gradual phenotypic conversion associated with immortalization of cultured human mammary epithelial cells. Mol Biol Cell 8:2391–2405
Klingelhutz AJ, Foster SA, McDougall JK (1996) Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79–82
Stoppler H, Hartmann DP, Sherman L, Schlegel R (1997) The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J Biol Chem 272:13332–13337
Howie HL, Katzenellenbogen RA, Galloway DA (2009) Papillomavirus E6 proteins. Virology 384:324–334
Band V, Zajchowski D, Kulesa V, Sager R (1990) Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci USA 87:463–467
Shay JW, Wright WE, Brasiskyte D, Van der Haegen BA (1993) E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene 8:1407–1413
Garbe JC, Vrba L, Sputova K, Fuchs L, Novak P, Brothman AR, Jackson M, Chin K, LaBarge MA, Watts G, Futscher BW, Stampfer MR (2014) Immortalization of normal human mammary epithelial cells in two steps by direct targeting of senescence barriers does not require gross genomic alterations. Cell Cycle 13:3423–3435
Bryson BL, Junk DJ, Cipriano R, Jackson MW (2016) STAT3-mediated SMAD3 activation underlies oncostatin M-induced senescence. Cell Cycle 16:319–334
Garbe JC, Holst CR, Bassett E, Tlsty T, Stampfer MR (2007) Inactivation of p53 function in cultured human mammary epithelial cells turns the telomere-length dependent senescence barrier from agonescence into crisis. Cell Cycle 6:1927–1936
Ben-Porath I, Weinberg RA (2005) The signals and pathways activating cellular senescence. Int J Biochem Cell Biol 37:961–976
Kang MK, Kameta A, Shin KH, Baluda MA, Park NH (2004) Senescence occurs with hTERT repression and limited telomere shortening in human oral keratinocytes cultured with feeder cells. J Cell Physiol 199:364–370
Huschtscha LI, Noble JR, Neumann AA, Moy EL, Barry P, Melki JR, Clark SJ, Reddel RR (1998) Loss of p16INK4 expression by methylation is associated with lifespan extension of human mammary epithelial cells. Cancer Res 58:3508–3512
Ramirez RD, Herbert BS, Vaughan MB, Zou Y, Gandia K, Morales CP, Wright WE, Shay JW (2003) Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells. Oncogene 22:433–444
Miller J, Dakic A, Chen R, Palechor-Ceron N, Dai Y, Kallakury B, Schlegel R, Liu X (2013) HPV16 E7 protein and hTERT proteins defective for telomere maintenance cooperate to immortalize human keratinocytes. PLoS Pathog 9:e1003284
Dimri GP, Martinez JL, Jacobs JJ, Keblusek P, Itahana K, Van Lohuizen M, Campisi J, Wazer DE, Band V (2002) The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res 62:4736–4745
Dabelsteen S, Hercule P, Barron P, Rice M, Dorsainville G, Rheinwald JG (2009) Epithelial cells derived from human embryonic stem cells display p16INK4A senescence, hypermotility, and differentiation properties shared by many P63+ somatic cell types. Stem Cells 27:1388–1399
Sedic M, Skibinski A, Brown N, Gallardo M, Mulligan P, Martinez P, Keller PJ, Glover E, Richardson AL, Cowan J, Toland AE, Ravichandran K, Riethman H, Naber SP, Naar AM, Blasco MA, Hinds PW, Kuperwasser C (2015) Haploinsufficiency for BRCA1 leads to cell-type-specific genomic instability and premature senescence. Nat Commun 6:7505
Maurelli R, Tinaburri L, Gangi F, Bondanza S, Severi AL, Scarponi C, Albanesi C, Mesiti G, Guerra L, Capogrossi MC, Dellambra E (2016) The role of oncogenic Ras in human skin tumorigenesis depends on the clonogenic potential of the founding keratinocytes. J Cell Sci 129:1003–1017
Olsen CL, Gardie B, Yaswen P, Stampfer MR (2002) Raf-1-induced growth arrest in human mammary epithelial cells is p16-independent and is overcome in immortal cells during conversion. Oncogene 21:6328–6339
Jarrard DF, Sarkar S, Shi Y, Yeager TR, Magrane G, Kinoshita H, Nassif N, Meisner L, Newton MA, Waldman FM, Reznikoff CA (1999) p16/pRb pathway alterations are required for bypassing senescence in human prostate epithelial cells. Cancer Res 59:2957–2964
Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B, von Werder A, Enders GH, Opitz OG, Rustgi AK (2003) Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res 1:729–738
Schwarze SR, Shi Y, Fu VX, Watson PA, Jarrard DF (2001) Role of cyclin-dependent kinase inhibitors in the growth arrest at senescence in human prostate epithelial and uroepithelial cells. Oncogene 20:8184–8192
de Carne Trecesson S, Guillemin Y, Belanger A, Bernard AC, Preisser L, Ravon E, Gamelin E, Juin P, Barre B, Coqueret O (2011) Escape from p21-mediated oncogene-induced senescence leads to cell dedifferentiation and dependence on anti-apoptotic Bcl-xL and MCL1 proteins. J Biol Chem 286:12825–12838
Cheung M, Testa JR (2013) Diverse mechanisms of AKT pathway activation in human malignancy. Curr Cancer Drug Targets 13:234–244
Verma S, Goyal S, Jamal S, Singh A, Grover A (2016) Hsp90: friends, clients and natural foes. Biochimie 127:227–240
Friguet B (2006) Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett 580:2910–2916
Tsakiri EN, Trougakos IP (2015) The amazing ubiquitin-proteasome system: structural components and implication in aging. Int Rev Cell Mol Biol 314:171–237
Koga H, Kaushik S, Cuervo AM (2011) Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev 10:205–215
Kaushik S, Cuervo AM (2015) Proteostasis and aging. Nat Med 21:1406–1415
Pluquet O, Pourtier A, Abbadie C (2014) The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol 308:C415–C425
Zhu B, Ferry CH, Markell LK, Blazanin N, Glick AB, Gonzalez FJ, Peters JM (2014) The nuclear receptor peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) promotes oncogene-induced cellular senescence through repression of endoplasmic reticulum stress. J Biol Chem 289:20102–20119
Liu J, Huang K, Cai GY, Chen XM, Yang JR, Lin LR, Yang J, Huo BG, Zhan J, He YN (2014) Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal 26:110–121
Jackson MP, Hewitt EW (2016) Cellular proteostasis: degradation of misfolded proteins by lysosomes. Essays Biochem 60:173–180
Ariosa AR, Klionsky DJ (2016) Autophagy core machinery: overcoming spatial barriers in neurons. J Mol Med (Berl) 94:1217–1227
Mijaljica D, Prescott M, Devenish RJ (2011) Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy 7:673–682
Cuervo AM, Wong E (2014) Chaperone-mediated autophagy: roles in disease and aging. Cell Res 24:92–104
Xilouri M, Stefanis L (2016) Chaperone mediated autophagy in aging: starve to prosper. Ageing Res Rev 32:13–21
Grasso D, Vaccaro MI (2014) Macroautophagy and the oncogene-induced senescence. Front Endocrinol (Lausanne) 5:157
Hoare M, Young AR, Narita M (2011) Autophagy in cancer: having your cake and eating it. Semin Cancer Biol 21:397–404
Baisantry A, Bhayana S, Wrede C, Hegermann J, Haller H, Melk A, Schmitt R (2016) The impact of autophagy on the development of senescence in primary tubular epithelial cells. Cell Cycle 15:2973–2979
Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y (2010) Autophagy mediates the process of cellular senescence characterizing bile duct damages in primary biliary cirrhosis. Lab Invest 90:835–843
Fujii S, Hara H, Araya J, Takasaka N, Kojima J, Ito S, Minagawa S, Yumino Y, Ishikawa T, Numata T, Kawaishi M, Hirano J, Odaka M, Morikawa T, Nishimura S, Nakayama K, Kuwano K (2012) Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology 1:630–641
Deruy E, Gosselin K, Vercamer C, Martien S, Bouali F, Slomianny C, Bertout J, Bernard D, Pourtier A, Abbadie C (2010) MnSOD upregulation induces autophagic programmed cell death in senescent keratinocytes. PLoS One 5:e12712
Elgendy M, Sheridan C, Brumatti G, Martin SJ (2011) Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell 42:23–35
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Muller M (2009) Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal 11:59–98
Bernard D, Gosselin K, Monte D, Vercamer C, Bouali F, Pourtier A, Vandenbunder B, Abbadie C (2004) Involvement of Rel/NF-κB transcription factors in keratinocyte senescence. Cancer Res 64:472–481
Cuenda A, Rousseau S (2007) p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773:1358–1375
Garcia-Cano J, Roche O, Cimas FJ, Pascual-Serra R, Ortega-Muelas M, Fernandez-Aroca DM, Sanchez-Prieto R (2016) p38MAPK and chemotherapy: we always need to hear both sides of the story. Front Cell Dev Biol 4:69
Reinhardt HC, Yaffe MB (2009) Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol 21:245–255
Debacq-Chainiaux F, Boilan E, Dedessus Le Moutier J, Weemaels G, Toussaint O (2010) p38(MAPK) in the senescence of human and murine fibroblasts. Adv Exp Med Biol 694:126–137
Caravia L, Dudau M, Gherghiceanu M, Tanase C, Enciu AM (2015) Could caveolae be acting as warnings of mitochondrial ageing? Mech Ageing Dev 146–148:81–87
Shin DM, Jeon JH, Kim CW, Cho SY, Lee HJ, Jang GY, Jeong EM, Lee DS, Kang JH, Melino G, Park SC, Kim IG (2008) TGFbeta mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. Faseb J 22:2498–2507
Li H, Sekine M, Seng S, Avraham S, Avraham HK (2009) BRCA1 interacts with Smad3 and regulates Smad3-mediated TGF-beta signaling during oxidative stress responses. PLoS One 4:e7091
Mirochnik Y, Veliceasa D, Williams L, Maxwell K, Yemelyanov A, Budunova I, Volpert OV (2012) Androgen receptor drives cellular senescence. PLoS One 7:e31052
Caldecott KW (2008) Single-strand break repair and genetic disease. Nat Rev Genet 9:619–631
Caldecott KW (2014) DNA single-strand break repair. Exp Cell Res 329:2–8
Kubota Y, Takanami T, Higashitani A, Horiuchi S (2009) Localization of X-ray cross complementing gene 1 protein in the nuclear matrix is controlled by casein kinase II-dependent phosphorylation in response to oxidative damage. DNA Repair (Amst) 8:953–960
Parsons JL, Dianova II, Finch D, Tait PS, Strom CE, Helleday T, Dianov GL (2010) XRCC1 phosphorylation by CK2 is required for its stability and efficient DNA repair. DNA Repair (Amst) 9:835–841
Wei L, Nakajima S, Hsieh CL, Kanno S, Masutani M, Levine AS, Yasui A, Lan L (2013) Damage response of XRCC1 at sites of DNA single strand breaks is regulated by phosphorylation and ubiquitylation after degradation of poly(ADP-ribose). J Cell Sci 126:4414–4423
Rayess H, Wang MB, Srivatsan ES (2012) Cellular senescence and tumor suppressor gene p16. Int J Cancer 130:1715–1725
Overhoff MG, Garbe JC, Koh J, Stampfer MR, Beach DH, Bishop CL (2014) Cellular senescence mediated by p16INK4A-coupled miRNA pathways. Nucleic Acids Res 42:1606–1618
Vijayachandra K, Higgins W, Lee J, Glick A (2009) Induction of p16ink4a and p19ARF by TGFbeta1 contributes to growth arrest and senescence response in mouse keratinocytes. Mol Carcinog 48:181–186
Plymate SR, Haugk KH, Sprenger CC, Nelson PS, Tennant MK, Zhang Y, Oberley LW, Zhong W, Drivdahl R, Oberley TD (2003) Increased manganese superoxide dismutase (SOD-2) is part of the mechanism for prostate tumor suppression by Mac25/insulin-like growth factor binding-protein-related protein-1. Oncogene 22:1024–1034
Bernard D, Slomianny C, Vandenbunder B, Abbadie C (2001) cRel induces mitochondrial alterations in correlation with proliferation arrest. Free Radic Biol Med 31:943–953
Mowla SN, Perkins ND, Jat PS (2013) Friend or foe: emerging role of nuclear factor kappa-light-chain-enhancer of activated B cells in cell senescence. Onco Targets Ther 6:1221–1229
Ohanna M, Giuliano S, Bonet C, Imbert V, Hofman V, Zangari J, Bille K, Robert C, Bressac-de Paillerets B, Hofman P, Rocchi S, Peyron JF, Lacour JP, Ballotti R, Bertolotto C (2011) Senescent cells develop a PARP-1 and nuclear factor-κB-associated secretome (PNAS). Genes Dev 25:1245–1261
Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C (2010) A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-κB activation. Mol Cell 40:63–74
Freund A, Patil CK, Campisi J (2011) p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J 30:1536–1548
Walen KH (2004) Spontaneous cell transformation: karyoplasts derived from multinucleated cells produce new cell growth in senescent human epithelial cell cultures. In Vitro Cell Dev Biol Anim 40:150–158
Sundaram M, Guernsey DL, Rajaraman MM, Rajaraman R (2004) Neosis: a novel type of cell division in cancer. Cancer Biol Ther 3:207–218
Rohnalter V, Roth K, Finkernagel F, Adhikary T, Obert J, Dorzweiler K, Bensberg M, Muller-Brusselbach S, Muller R (2015) A multi-stage process including transient polyploidization and EMT precedes the emergence of chemoresistent ovarian carcinoma cells with a dedifferentiated and pro-inflammatory secretory phenotype. Oncotarget 6:40005–40025
Jonchere B, Vetillard A, Toutain B, Lam D, Bernard AC, Henry C, De Carne Trecesson S, Gamelin E, Juin P, Guette C, Coqueret O (2015) Irinotecan treatment and senescence failure promote the emergence of more transformed and invasive cells that depend on anti-apoptotic Mcl-1. Oncotarget 6:409–426
Novak P, Jensen TJ, Garbe JC, Stampfer MR, Futscher BW (2009) Stepwise DNA methylation changes are linked to escape from defined proliferation barriers and mammary epithelial cell immortalization. Cancer Res 69:5251–5258
Holst CR, Nuovo GJ, Esteller M, Chew K, Baylin SB, Herman JG, Tlsty TD (2003) Methylation of p16(INK4a) promoters occurs in vivo in histologically normal human mammary epithelia. Cancer Res 63:1596–1601
Erenpreisa J, Salmina K, Huna A, Kosmacek EA, Cragg MS, Ianzini F, Anisimov AP (2011) Polyploid tumour cells elicit paradiploid progeny through depolyploidizing divisions and regulated autophagic degradation. Cell Biol Int 35:687–695
Puig PE, Guilly MN, Bouchot A, Droin N, Cathelin D, Bouyer F, Favier L, Ghiringhelli F, Kroemer G, Solary E, Martin F, Chauffert B (2008) Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol Int 32:1031–1043
Malaquin N, Martinez A, Rodier F (2016) Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp Gerontol 82:39–49
Sasaki M, Ikeda H, Sato Y, Nakanuma Y (2008) Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study. Free Radic Res 42:625–632
Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y (2010) Modulation of the microenvironment by senescent biliary epithelial cells may be involved in the pathogenesis of primary biliary cirrhosis. J Hepatol 53:318–325
Shivshankar P, Brampton C, Miyasato S, Kasper M, Thannickal VJ, Le Saux CJ (2012) Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am J Respir Cell Mol Biol 47:28–36
Aoshiba K, Tsuji T, Kameyama S, Itoh M, Semba S, Yamaguchi K, Nakamura H (2013) Senescence-associated secretory phenotype in a mouse model of bleomycin-induced lung injury. Exp Toxicol Pathol 65:1053–1062
Vital P, Castro P, Tsang S, Ittmann M (2014) The senescence-associated secretory phenotype promotes benign prostatic hyperplasia. Am J Pathol 184:721–731
Song JH, Kandasamy K, Zemskova M, Lin YW, Kraft AS (2011) The BH3 mimetic ABT-737 induces cancer cell senescence. Cancer Res 71:506–515
Marazita MC, Dugour A, Marquioni-Ramella MD, Figueroa JM, Suburo AM (2016) Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biol 7:78–87
Cao S, Walker GB, Wang X, Cui JZ, Matsubara JA (2013) Altered cytokine profiles of human retinal pigment epithelium: oxidant injury and replicative senescence. Mol Vis 19:718–728
Ivison SM, Wang C, Himmel ME, Sheridan J, Delano J, Mayer ML, Yao Y, Kifayet A, Steiner TS (2010) Oxidative stress enhances IL-8 and inhibits CCL20 production from intestinal epithelial cells in response to bacterial flagellin. Am J Physiol Gastrointest Liver Physiol 299:G733–G741
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6:2853–2868
Zurgil U, Ben-Ari A, Atias K, Isakov N, Apte R, Livneh E (2014) PKCeta promotes senescence induced by oxidative stress and chemotherapy. Cell Death Dis 5:e1531
Kato M, Ishizaki A, Hellman U, Wernstedt C, Kyogoku M, Miyazono K, Heldin CH, Funa K (1995) A human keratinocyte cell line produces two autocrine growth inhibitors, transforming growth factor-beta and insulin-like growth factor binding protein-6, in a calcium- and cell density-dependent manner. J Biol Chem 270:12373–12379
Akiel M, Rajasekaran D, Gredler R, Siddiq A, Srivastava J, Robertson C, Jariwala NH, Fisher PB, Sarkar D (2014) Emerging role of insulin-like growth factor-binding protein 7 in hepatocellular carcinoma. J Hepatocell Carcinoma 1:9–19
Swisshelm K, Ryan K, Tsuchiya K, Sager R (1995) Enhanced expression of an insulin growth factor-like binding protein (mac25) in senescent human mammary epithelial cells and induced expression with retinoic acid. Proc Natl Acad Sci USA 92:4472–4476
Lo Pez-Bermejo A, Buckway CK, Devi GR, Hwa V, Plymate SR, Oh Y, Rosenfeld RG (2000) Characterization of insulin-like growth factor-binding protein-related proteins (IGFBP-rPs) 1, 2, and 3 in human prostate epithelial cells: potential roles for IGFBP-rP1 and 2 in senescence of the prostatic epithelium. Endocrinology 141:4072–4080
Benatar T, Yang W, Amemiya Y, Evdokimova V, Kahn H, Holloway C, Seth A (2012) IGFBP7 reduces breast tumor growth by induction of senescence and apoptosis pathways. Breast Cancer Res Treat 133:563–573
Munoz-Espin D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15:482–496
Jun JI, Lau LF (2010) Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2:627–631
Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31:722–733
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134:657–667
Evangelou K, Lougiakis N, Rizou SV, Kotsinas A, Kletsas D, Munoz-Espin D, Kastrinakis NG, Pouli N, Marakos P, Townsend P, Serrano M, Bartek J, Gorgoulis VG (2017) Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 16:192–197
Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114:1299–1307
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530:184–189
Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF (2004) Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65:510–520
Liu J, Yang JR, He YN, Cai GY, Zhang JG, Lin LR, Zhan J, Zhang JH, Xiao HS (2012) Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl Res 159:454–463
Li ZY, Chen ZL, Zhang T, Wei C, Shi WY (2016) TGF-beta and NF-κB signaling pathway crosstalk potentiates corneal epithelial senescence through an RNA stress response. Aging (Albany NY) 8:2337–2354
Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T (2009) DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8:311–323
Berkenkamp B, Susnik N, Baisantry A, Kuznetsova I, Jacobi C, Sorensen-Zender I, Broecker V, Haller H, Melk A, Schmitt R (2014) In vivo and in vitro analysis of age-associated changes and somatic cellular senescence in renal epithelial cells. PLoS One 9:e88071
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92:9363–9367
Choi J, Shendrik I, Peacocke M, Peehl D, Buttyan R, Ikeguchi EF, Katz AE, Benson MC (2000) Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology 56:160–166
Castro P, Giri D, Lamb D, Ittmann M (2003) Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate 55:30–38
Castro P, Xia C, Gomez L, Lamb DJ, Ittmann M (2004) Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate 60:153–159
Olinski R, Zastawny TH, Foksinski M, Barecki A, Dizdaroglu M (1995) DNA base modifications and antioxidant enzyme activities in human benign prostatic hyperplasia. Free Radic Biol Med 18:807–813
Sasaki M, Nakanuma Y (2015) Cellular senescence in biliary pathology. Special emphasis on expression of a polycomb group protein EZH2 and a senescent marker p16INK4a in bile ductular tumors and lesions. Histol Histopathol 30:267–275
Yamaguchi J, Sasaki M, Harada K, Zen Y, Sato Y, Ikeda H, Itatsu K, Yokoyama Y, Ando H, Ohta T, Kubota A, Shimizu K, Nimura Y, Nagino M, Nakanuma Y (2009) Papillary hyperplasia of the gallbladder in pancreaticobiliary maljunction represents a senescence-related lesion induced by lysolecithin. Lab Invest 89:1018–1031
Radisky DC, Santisteban M, Berman HK, Gauthier ML, Frost MH, Reynolds CA, Vierkant RA, Pankratz VS, Visscher DW, Tlsty TD, Hartmann LC (2011) p16(INK4a) expression and breast cancer risk in women with atypical hyperplasia. Cancer Prev Res (Phila) 4:1953–1960
Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M (2005) Tumour biology: senescence in premalignant tumours. Nature 436:642
Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA (2003) Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev 17:3112–3126
Carriere C, Gore AJ, Norris AM, Gunn JR, Young AL, Longnecker DS, Korc M (2011) Deletion of Rb accelerates pancreatic carcinogenesis by oncogenic Kras and impairs senescence in premalignant lesions. Gastroenterology 141:1091–1101
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14:644–658
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15:428–435
Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I, Krizhanovsky V (2015) Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 7:11190
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22:78–83
Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, Papaconstantinou J, Taylor RN (2013) Senescence of primary amniotic cells via oxidative DNA damage. PLoS One 8:e83416
Behnia F, Peltier MR, Saade GR, Menon R (2015) Environmental pollutant polybrominated diphenyl ether, a flame retardant, induces primary amnion cell senescence. Am J Reprod Immunol 74:398–406
Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R (2015) Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One 10:e0137188
Sasaki M, Ikeda H, Haga H, Manabe T, Nakanuma Y (2005) Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol 205:451–459
Sasaki M, Ikeda H, Sato Y, Nakanuma Y (2006) Decreased expression of Bmi1 is closely associated with cellular senescence in small bile ducts in primary biliary cirrhosis. Am J Pathol 169:831–845
Sasaki M, Ikeda H, Yamaguchi J, Nakada S, Nakanuma Y (2008) Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence. Hepatology 48:186–195
Demetris AJ, Markus BH, Saidman S, Fung JJ, Makowka L, Graner S, Duquesnoy R, Starzl TE (1988) Isolation and primary cultures of human intrahepatic bile ductular epithelium. In Vitro Cell Dev Biol 24:464–470
Lunz JG 3rd, Contrucci S, Ruppert K, Murase N, Fung JJ, Starzl TE, Demetris AJ (2001) Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21(WAF1/Cip1) as a disease marker and the influence of immunosuppressive drugs. Am J Pathol 158:1379–1390
Qin XY, Fukuda T, Yang L, Zaha H, Akanuma H, Zeng Q, Yoshinaga J, Sone H (2012) Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer Biol Ther 13:296–306
Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d’Adda di Fagagna F (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444:638–642
Maund SL, Shi L, Cramer SD (2013) A role for interleukin-1 alpha in the 1,25 dihydroxyvitamin D3 response in mammary epithelial cells. PLoS One 8:e81367
Trost TM, Lausch EU, Fees SA, Schmitt S, Enklaar T, Reutzel D, Brixel LR, Schmidtke P, Maringer M, Schiffer IB, Heimerdinger CK, Hengstler JG, Fritz G, Bockamp EO, Prawitt D, Zabel BU, Spangenberg C (2005) Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res 65:840–849
Elmore LW, Rehder CW, Di X, McChesney PA, Jackson-Cook CK, Gewirtz DA, Holt SE (2002) Adriamycin-induced senescence in breast tumor cells involves functional p53 and telomere dysfunction. J Biol Chem 277:35509–35515
Yaar M, Eller MS, Panova I, Kubera J, Wee LH, Cowan KH, Gilchrest BA (2007) Telomeric DNA induces apoptosis and senescence of human breast carcinoma cells. Breast Cancer Res 9:R13
Tato-Costa J, Casimiro S, Pacheco T, Pires R, Fernandes A, Alho I, Pereira P, Costa P, Castelo HB, Ferreira J, Costa L (2016) Therapy-induced cellular senescence induces epithelial-to-mesenchymal transition and increases invasiveness in rectal cancer. Clin Colorectal Cancer 15(170–178):e3
Dalerba P, Guiducci C, Poliani PL, Cifola I, Parenza M, Frattini M, Gallino G, Carnevali I, Di Giulio I, Andreola S, Lombardo C, Rivoltini L, Schweighoffer T, Belli F, Colombo MP, Parmiani G, Castelli C (2005) Reconstitution of human telomerase reverse transcriptase expression rescues colorectal carcinoma cells from in vitro senescence: evidence against immortality as a constitutive trait of tumor cells. Cancer Res 65:2321–2329
te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP (2002) DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 62:1876–1883
De Simone C, Ferranti P, Picariello G, Scognamiglio I, Dicitore A, Addeo F, Chianese L, Stiuso P (2011) Peptides from water buffalo cheese whey induced senescence cell death via ceramide secretion in human colon adenocarcinoma cell line. Mol Nutr Food Res 55:229–238
Han Z, Wei W, Dunaway S, Darnowski JW, Calabresi P, Sedivy J, Hendrickson EA, Balan KV, Pantazis P, Wyche JH (2002) Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem 277:17154–17160
Rudolf E, John S, Cervinka M (2012) Irinotecan induces senescence and apoptosis in colonic cells in vitro. Toxicol Lett 214:1–8
Gao J, Wang HL, Shreve A, Iyer R (2010) Fullerene derivatives induce premature senescence: a new toxicity paradigm or novel biomedical applications. Toxicol Appl Pharmacol 244:130–143
Rockwell GA, Johnson G, Sibatani A (1987) In vitro senescence of human keratinocyte cultures. Cell Struct Funct 12:539–548
Munro J, Stott FJ, Vousden KH, Peters G, Parkinson EK (1999) Role of the alternative INK4A proteins in human keratinocyte senescence: evidence for the specific inactivation of p16INK4A upon immortalization. Cancer Res 59:2516–2521
Shen ZY, Xu LY, Li EM, Shen J, Zheng RM, Cai WJ, Zeng Y (2002) Immortal phenotype of the esophageal epithelial cells in the process of immortalization. Int J Mol Med 10:641–646
Zhang ZF, Zhang J, Hui YN, Zheng MH, Liu XP, Kador PF, Wang YS, Yao LB, Zhou J (2011) Up-regulation of NDRG2 in senescent lens epithelial cells contributes to age-related cataract in human. PLoS One 6:e26102
Wang J, Feng H, Huang XQ, Xiang H, Mao YW, Liu JP, Yan Q, Liu WB, Liu Y, Deng M, Gong L, Sun S, Luo C, Liu SJ, Zhang XJ, Liu Y, Li DW (2005) Human telomerase reverse transcriptase immortalizes bovine lens epithelial cells and suppresses differentiation through regulation of the ERK signaling pathway. J Biol Chem 280:22776–22787
Gao ZX, Song XL, Li SS, Lai XR, Yang YL, Yang G, Li ZJ, Cui YH, Pan HW (2016) Assessment of DNA damage and cell senescence in corneal epithelial cells exposed to airborne particulate matter (PM2.5) collected in Guangzhou, China. Invest Ophthalmol Vis Sci 57:3093–3102
Zhuge CC, Xu JY, Zhang J, Li W, Li P, Li Z, Chen L, Liu X, Shang P, Xu H, Lu Y, Wang F, Lu L, Xu GT (2014) Fullerenol protects retinal pigment epithelial cells from oxidative stress-induced premature senescence via activating SIRT1. Invest Ophthalmol Vis Sci 55:4628–4638
Yu AL, Fuchshofer R, Kook D, Kampik A, Bloemendal H, Welge-Lussen U (2009) Subtoxic oxidative stress induces senescence in retinal pigment epithelial cells via TGF-beta release. Invest Ophthalmol Vis Sci 50:926–935
Yu AL, Lorenz RL, Haritoglou C, Kampik A, Welge-Lussen U (2009) Biological effects of native and oxidized low-density lipoproteins in cultured human retinal pigment epithelial cells. Exp Eye Res 88:495–503
Demidenko ZN, Blagosklonny MV (2008) Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle 7:3355–3361
Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A, Welge-Lussen UC (2008) The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci 49:1712–1720
Zhu D, Wu J, Spee C, Ryan SJ, Hinton DR (2009) BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J Biol Chem 284:9529–9539
Honda S, Weigel A, Hjelmeland LM, Handa JT (2001) Induction of telomere shortening and replicative senescence by cryopreservation. Biochem Biophys Res Commun 282:493–498
Honda S, Hjelmeland LM, Handa JT (2002) Senescence associated beta galactosidase activity in human retinal pigment epithelial cells exposed to mild hyperoxia in vitro. Br J Ophthalmol 86:159–162
Matsunaga H, Handa JT, Aotaki-Keen A, Sherwood SW, West MD, Hjelmeland LM (1999) Beta-galactosidase histochemistry and telomere loss in senescent retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 40:197–202
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Rawes V, Kipling D, Kill IR, Faragher RG (1997) The kinetics of senescence in retinal pigmented epithelial cells: a test for the telomere hypothesis of ageing? Biochemistry (Mosc) 62:1291–1295
Lu Y, Wang J, Dapeng C, Wu D, Cai G, Chen X (2016) Bioinformatics analysis of proteomics profiles in senescent human primary proximal tubule epithelial cells. BMC Nephrol 17:39
Jennings P, Koppelstaetter C, Aydin S, Abberger T, Wolf AM, Mayer G, Pfaller W (2007) Cyclosporine A induces senescence in renal tubular epithelial cells. Am J Physiol Renal Physiol 293:F831–F838
Melk A, Schmidt BM, Vongwiwatana A, Rayner DC, Halloran PF (2005) Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 5:1375–1382
Ferlicot S, Durrbach A, Ba N, Desvaux D, Bedossa P, Paradis V (2003) The role of replicative senescence in chronic allograft nephropathy. Hum Pathol 34:924–928
Joosten SA, van Ham V, Nolan CE, Borrias MC, Jardine AG, Shiels PG, van Kooten C, Paul LC (2003) Telomere shortening and cellular senescence in a model of chronic renal allograft rejection. Am J Pathol 162:1305–1312
Chkhotua AB, Altimari A, Gabusi E, D’Errico A, Stefoni S, Chieco P, Yakubovich M, Vienken J, Yussim A, Grigioni WF (2003) Increased expression of p21 (WAF1/CIP1) cyclin-dependent kinase (CDK) inhibitor gene in chronic allograft nephropathy correlates with the number of acute rejection episodes. Transpl Int 16:502–506
Chkhotua AB, Gabusi E, Altimari A, D’Errico A, Yakubovich M, Vienken J, Stefoni S, Chieco P, Yussim A, Grigioni WF (2003) Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am J Kidney Dis 41:1303–1313
Park JY, Park SH, Weiss RH (2009) Disparate effects of roscovitine on renal tubular epithelial cell apoptosis and senescence: implications for autosomal dominant polycystic kidney disease. Am J Nephrol 29:509–515
Takaki A, Jimi S, Segawa M, Iwasaki H (2004) Cadmium-induced nephropathy in rats is mediated by expression of senescence-associated beta-galactosidase and accumulation of mitochondrial DNA deletion. Ann N Y Acad Sci 1011:332–338
Bodas M, Van Westphal C, Carpenter-Thompson R, Mohanty DK, Vij N (2016) Nicotine exposure induces bronchial epithelial cell apoptosis and senescence via ROS mediated autophagy-impairment. Free Radic Biol Med 97:441–453
Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I (2015) Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. Faseb J 29:2912–2929
Muller M (2006) Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic Biol Med 41:1670–1677
Tsuji T, Aoshiba K, Nagai A (2004) Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol 31:643–649
Tsuji T, Aoshiba K, Nagai A (2006) Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 174:886–893
Parker SM, Goriwiec MR, Borthwick LA, Johnson G, Ward C, Lordan JL, Corris PA, Saretzki GC, Fisher AJ (2008) Airway epithelial cell senescence in the lung allograft. Am J Transplant 8:1544–1549
Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K (2011) Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300:L391–L401
Aoshiba K, Tsuji T, Nagai A (2003) Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J 22:436–443
Kim CO, Huh AJ, Han SH, Kim JM (2012) Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch Gerontol Geriatr 54:e35–e41
Kang MK, Guo W, Park NH (1998) Replicative senescence of normal human oral keratinocytes is associated with the loss of telomerase activity without shortening of telomeres. Cell Growth Differ 9:85–95
Kang MK, Kim RH, Shin KH, Zhong W, Faull KF, Park NH (2005) Senescence-associated decline in the intranuclear accumulation of hOGG1-alpha and impaired 8-oxo-dG repair activity in senescing normal human oral keratinocytes in vivo. Exp Cell Res 310:186–195
You YO, Lee G, Min BM (2000) Retinoic acid extends the in vitro life span of normal human oral keratinocytes by decreasing p16(INK4A) expression and maintaining telomerase activity. Biochem Biophys Res Commun 268:268–274
Kim RH, Lieberman MB, Lee R, Shin KH, Mehrazarin S, Oh JE, Park NH, Kang MK (2010) Bmi-1 extends the life span of normal human oral keratinocytes by inhibiting the TGF-beta signaling. Exp Cell Res 316:2600–2608
Kim RH, Lee RS, Williams D, Bae S, Woo J, Lieberman M, Oh JE, Dong Q, Shin KH, Kang MK, Park NH (2011) Bisphosphonates induce senescence in normal human oral keratinocytes. J Dent Res 90:810–816
Seki Y, Koizumi H, Koizuka I, Takakuwa T, Tsutsumi K (2000) Nuclear expression of the p16CDKN2 gene product during senescence of human pharyngeal epithelial cells. Auris Nasus Larynx 27:147–151
Frame FM, Savoie H, Bryden F, Giuntini F, Mann VM, Simms MS, Boyle RW, Maitland NJ (2016) Mechanisms of growth inhibition of primary prostate epithelial cells following gamma irradiation or photodynamic therapy include senescence, necrosis, and autophagy, but not apoptosis. Cancer Med 5:61–73
Schwarze SR, Fu VX, Desotelle JA, Kenowski ML, Jarrard DF (2005) The identification of senescence-specific genes during the induction of senescence in prostate cancer cells. Neoplasia 7:816–823
Pernicova Z, Slabakova E, Kharaishvili G, Bouchal J, Kral M, Kunicka Z, Machala M, Kozubik A, Soucek K (2011) Androgen depletion induces senescence in prostate cancer cells through down-regulation of Skp2. Neoplasia 13:526–536
Axanova LS, Chen YQ, McCoy T, Sui G, Cramer SD (2010) 1,25-dihydroxyvitamin D(3) and PI3K/AKT inhibitors synergistically inhibit growth and induce senescence in prostate cancer cells. Prostate 70:1658–1671
Reznikoff CA, Yeager TR, Belair CD, Savelieva E, Puthenveettil JA, Stadler WM (1996) Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res 56:2886–2890
Chang BD, Broude EV, Dokmanovic M, Zhu H, Ruth A, Xuan Y, Kandel ES, Lausch E, Christov K, Roninson IB (1999) A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res 59:3761–3767
Chen YL, Chen YJ, Tsai WH, Ko YC, Chen JY, Lin SF (2009) The Epstein-Barr virus replication and transcription activator, Rta/BRLF1, induces cellular senescence in epithelial cells. Cell Cycle 8:58–65
Basu D, Reyes-Mugica M, Rebbaa A (2012) Role of the beta catenin destruction complex in mediating chemotherapy-induced senescence-associated secretory phenotype. PLoS One 7:e52188
Basu N, Saha S, Khan I, Ramachandra SG, Visweswariah SS (2014) Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem 289:581–593
Wiel C, Gras B, Vindrieux D, Warnier M, Gitenay D, Le Calve B, Ferrand M, Augert A, Bernard D (2016) Multidrug resistance protein 3 loss promotes tumor formation by inducing senescence escape. Oncogene 35:1596–1601
Oyama K, Okawa T, Nakagawa H, Takaoka M, Andl CD, Kim SH, Klein-Szanto A, Diehl JA, Herlyn M, El-Deiry W, Rustgi AK (2007) AKT induces senescence in primary esophageal epithelial cells but is permissive for differentiation as revealed in organotypic culture. Oncogene 26:2353–2364
Bhatia B, Tang S, Yang P, Doll A, Aumueller G, Newman RA, Tang DG (2005) Cell-autonomous induction of functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) contributes to replicative senescence of human prostate progenitor cells. Oncogene 24:3583–3595
Liu J, Yang JR, Chen XM, Cai GY, Lin LR, He YN (2015) Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am J Physiol Cell Physiol 308:C621–C630
Takasaka N, Araya J, Hara H, Ito S, Kobayashi K, Kurita Y, Wakui H, Yoshii Y, Yumino Y, Fujii S, Minagawa S, Tsurushige C, Kojima J, Numata T, Shimizu K, Kawaishi M, Kaneko Y, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K (2014) Autophagy induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. J Immunol 192:958–968
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444:633–637
Han S, Lu Q, Wang N (2016) Apr3 accelerates the senescence of human retinal pigment epithelial cells. Mol Med Rep 13:3121–3126
Lyros O, Rafiee P, Nie L, Medda R, Jovanovic N, Schmidt J, Mackinnon A, Venu N, Shaker R (2014) Dickkopf-1, the Wnt antagonist, is induced by acidic pH and mediates epithelial cellular senescence in human reflux esophagitis. Am J Physiol Gastrointest Liver Physiol 306:G557–G574
Zhang H, Chi Y, Gao K, Zhang X, Yao J (2015) p53 protein-mediated up-regulation of MAP kinase phosphatase 3 (MKP-3) contributes to the establishment of the cellular senescent phenotype through dephosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2). J Biol Chem 290:1129–1140
Angelini PD, Zacarias Fluck MF, Pedersen K, Parra-Palau JL, Guiu M, Bernado Morales C, Vicario R, Luque-Garcia A, Navalpotro NP, Giralt J, Canals F, Gomis RR, Tabernero J, Baselga J, Villanueva J, Arribas J (2013) Constitutive HER2 signaling promotes breast cancer metastasis through cellular senescence. Cancer Res 73:450–458
Kim JE, Shin JS, Moon JH, Hong SW, Jung DJ, Kim JH, Hwang IY, Shin YJ, Gong EY, Lee DH, Kim SM, Lee EY, Kim YS, Kim D, Hur D, Kim TW, Kim KP, Jin DH, Lee WJ (2016) Foxp3 is a key downstream regulator of p53-mediated cellular senescence. Oncogene 36:219–230
Wu Q, Jiang D, Matsuda JL, Ternyak K, Zhang B, Chu HW (2016) Cigarette smoke induces human airway epithelial senescence via growth differentiation factor 15 production. Am J Respir Cell Mol Biol 55:429–438
Chen Y, Yang L, Cui T, Pacyna-Gengelbach M, Petersen I (2015) HOPX is methylated and exerts tumour-suppressive function through Ras-induced senescence in human lung cancer. J Pathol 235:397–407
Wilson HM, Birnbaum RS, Poot M, Quinn LS, Swisshelm K (2002) Insulin-like growth factor binding protein-related protein 1 inhibits proliferation of MCF-7 breast cancer cells via a senescence-like mechanism. Cell Growth Differ 13:205–213
Xin H, Curry J, Johnstone RW, Nickoloff BJ, Choubey D (2003) Role of IFI 16, a member of the interferon-inducible p200-protein family, in prostate epithelial cellular senescence. Oncogene 22:4831–4840
Jia M, Souchelnytskyi N, Hellman U, O’Hare M, Jat PS, Souchelnytskyi S (2010) Proteome profiling of immortalization-to-senescence transition of human breast epithelial cells identified MAP2K3 as a senescence-promoting protein which is downregulated in human breast cancer. Proteomics Clin Appl 4:816–828
Cote M, Miller AD, Liu SL (2007) Human RON receptor tyrosine kinase induces complete epithelial-to-mesenchymal transition but causes cellular senescence. Biochem Biophys Res Commun 360:219–225
Chen Y, Wang J, Cai J, Sternberg P (2010) Altered mTOR signaling in senescent retinal pigment epithelium. Invest Ophthalmol Vis Sci 51:5314–5319
Turner-Ivey B, Manevich Y, Schulte J, Kistner-Griffin E, Jezierska-Drutel A, Liu Y, Neumann CA (2013) Role for Prdx1 as a specific sensor in redox-regulated senescence in breast cancer. Oncogene 32:5302–5314
Ruan JW, Liao YC, Lua I, Li MH, Hsu CY, Chen JH (2012) Human pituitary tumor-transforming gene 1 overexpression reinforces oncogene-induced senescence through CXCR2/p21 signaling in breast cancer cells. Breast Cancer Res 14:R106
Wen FC, Chang TW, Tseng YL, Lee JC, Chang MC (2014) hRAD9 functions as a tumor suppressor by inducing p21-dependent senescence and suppressing epithelial-mesenchymal transition through inhibition of Slug transcription. Carcinogenesis 35:1481–1490
Vijayachandra K, Lee J, Glick AB (2003) Smad3 regulates senescence and malignant conversion in a mouse multistage skin carcinogenesis model. Cancer Res 63:3447–3452
Zhang H, Teng Y, Kong Y, Kowalski PE, Cohen SN (2008) Suppression of human tumor cell proliferation by Smurf2-induced senescence. J Cell Physiol 215:613–620
Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y (2012) Increased expression of mitochondrial proteins associated with autophagy in biliary epithelial lesions in primary biliary cirrhosis. Liver Int 33:312–320
Xu Y, Zhang S, Niu H, Ye Y, Hu F, Chen S, Li X, Luo X, Jiang S, Liu Y, Chen Y, Li J, Xiang R, Li N (2015) STIM1 accelerates cell senescence in a remodeled microenvironment but enhances the epithelial-to-mesenchymal transition in prostate cancer. Sci Rep 5:11754
Wang Y, Wong MM, Zhang X, Chiu SK (2015) Ectopic AP4 expression induces cellular senescence via activation of p53 in long-term confluent retinal pigment epithelial cells. Exp Cell Res 339:135–146
Sugrue MM, Shin DY, Lee SW, Aaronson SA (1997) Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci USA 94:9648–9653
Sommer M, Poliak N, Upadhyay S, Ratovitski E, Nelkin BD, Donehower LA, Sidransky D (2006) DeltaNp63alpha overexpression induces downregulation of Sirt1 and an accelerated aging phenotype in the mouse. Cell Cycle 5:2005–2011
Keyes WM, Wu Y, Vogel H, Guo X, Lowe SW, Mills AA (2005) p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev 19:1986–1999
Lin S, Yang J, Elkahloun AG, Bandyopadhyay A, Wang L, Cornell JE, Yeh IT, Agyin J, Tomlinson G, Sun LZ (2012) Attenuation of TGF-beta signaling suppresses premature senescence in a p21-dependent manner and promotes oncogenic Ras-mediated metastatic transformation in human mammary epithelial cells. Mol Biol Cell 23:1569–1581
Bitler BG, Nicodemus JP, Li H, Cai Q, Wu H, Hua X, Li T, Birrer MJ, Godwin AK, Cairns P, Zhang R (2011) Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res 71:6184–6194
Brown NE, Jeselsohn R, Bihani T, Hu MG, Foltopoulou P, Kuperwasser C, Hinds PW (2012) Cyclin D1 activity regulates autophagy and senescence in the mammary epithelium. Cancer Res 72:6477–6489
Sarker RS, John-Schuster G, Bohla A, Mutze K, Burgstaller G, Bedford MT, Konigshoff M, Eickelberg O, Yildirim AO (2015) Coactivator-associated arginine methyltransferase-1 function in alveolar epithelial senescence and elastase-induced emphysema susceptibility. Am J Respir Cell Mol Biol 53:769–781
Hara H, Araya J, Takasaka N, Fujii S, Kojima J, Yumino Y, Shimizu K, Ishikawa T, Numata T, Kawaishi M, Saito K, Hirano J, Odaka M, Morikawa T, Hano H, Nakayama K, Kuwano K (2012) Involvement of creatine kinase B in cigarette smoke-induced bronchial epithelial cell senescence. Am J Respir Cell Mol Biol 46:306–312
Pan WW, Yi FP, Cao LX, Liu XM, Shen ZF, Bu YQ, Xu Y, Fan HY, Song FZ (2013) DAXX silencing suppresses mouse ovarian surface epithelial cell growth by inducing senescence and DNA damage. Gene 526:287–294
Alexander PB, Yuan L, Yang P, Sun T, Chen R, Xiang H, Chen J, Wu H, Radiloff DR, Wang XF (2015) EGF promotes mammalian cell growth by suppressing cellular senescence. Cell Res 25:135–138
Liu Z, Wang L, Yang J, Bandyopadhyay A, Kaklamani V, Wang S, Sun LZ (2016) Estrogen receptor alpha inhibits senescence-like phenotype and facilitates transformation induced by oncogenic ras in human mammary epithelial cells. Oncotarget 7:39097–39107
Smirnov A, Panatta E, Lena A, Castiglia D, Di Daniele N, Melino G, Candi E (2016) FOXM1 regulates proliferation, senescence and oxidative stress in keratinocytes and cancer cells. Aging (Albany NY) 8:1384–1397
Lu D, Rauhauser A, Li B, Ren C, McEnery K, Zhu J, Chaki M, Vadnagara K, Elhadi S, Jetten AM, Igarashi P, Attanasio M (2016) Loss of Glis2/NPHP7 causes kidney epithelial cell senescence and suppresses cyst growth in the Kif3a mouse model of cystic kidney disease. Kidney Int 89:1307–1323
Ikeda Y, Inagi R, Miyata T, Nagai R, Arai M, Miyashita M, Itokawa M, Fujita T, Nangaku M (2011) Glyoxalase I retards renal senescence. Am J Pathol 179:2810–2821
Gitenay D, Wiel C, Lallet-Daher H, Vindrieux D, Aubert S, Payen L, Simonnet H, Bernard D (2014) Glucose metabolism and hexosamine pathway regulate oncogene-induced senescence. Cell Death Dis 5:e1089
Gabai VL, Yaglom JA, Waldman T, Sherman MY (2009) Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol Cell Biol 29:559–569
O’Callaghan-Sunol C, Gabai VL, Sherman MY (2007) Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res 67:11779–11788
Chan KC, Ting CM, Chan PS, Lo MC, Lo KW, Curry JE, Smyth T, Lee AW, Ng WT, Tsao GS, Wong RN, Lung ML, Mak NK (2013) A novel Hsp90 inhibitor AT13387 induces senescence in EBV-positive nasopharyngeal carcinoma cells and suppresses tumor formation. Mol Cancer 12:128
Di K, Ling MT, Tsao SW, Wong YC, Wang X (2006) Id-1 modulates senescence and TGF-beta1 sensitivity in prostate epithelial cells. Biol Cell 98:523–533
Ohta K, Haraguchi N, Kano Y, Kagawa Y, Konno M, Nishikawa S, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Uemura M, Nishimura J, Hata T, Takemasa I, Mizushima T, Noguchi Y, Ozaki M, Kudo T, Sakai D, Satoh T, Fukami M, Ishii M, Yamamoto H, Doki Y, Mori M, Ishii H (2013) Depletion of JARID1B induces cellular senescence in human colorectal cancer. Int J Oncol 42:1212–1218
Lee KE, Bar-Sagi D (2010) Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell 18:448–458
Prencipe M, Fitzpatrick P, Gorman S, Tosetto M, Klinger R, Furlong F, Harrison M, O’Connor D, Roninson IB, O’Sullivan J, McCann A (2009) Cellular senescence induced by aberrant MAD2 levels impacts on paclitaxel responsiveness in vitro. Br J Cancer 101:1900–1908
Dvashi Z, Green Y, Pollack A (2014) TAK1 inhibition accelerates cellular senescence of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 55:5679–5686
Wu D, Yu S, Jia L, Zou C, Xu Z, Xiao L, Wong KB, Ng CF, Chan FL (2015) Orphan nuclear receptor TLX functions as a potent suppressor of oncogene-induced senescence in prostate cancer via its transcriptional co-regulation of the CDKN1A (p21(WAF1)(/)(CIP1)) and SIRT1 genes. J Pathol 236:103–115
Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, Kojima J, Shimizu K, Numata T, Kawaishi M, Odaka M, Morikawa T, Harada T, Nishimura SL, Kaneko Y, Nakayama K, Kuwano K (2015) PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11:547–559
Paget JA, Restall IJ, Daneshmand M, Mersereau JA, Simard MA, Parolin DA, Lavictoire SJ, Amin MS, Islam S, Lorimer IA (2012) Repression of cancer cell senescence by PKCiota. Oncogene 31:3584–3596
Gao Y, Zhao Y, Zhang J, Lu Y, Liu X, Geng P, Huang B, Zhang Y, Lu J (2016) The dual function of PRMT1 in modulating epithelial-mesenchymal transition and cellular senescence in breast cancer cells through regulation of ZEB1. Sci Rep 6:19874
Aird KM, Li H, Xin F, Konstantinopoulos PA, Zhang R (2014) Identification of ribonucleotide reductase M2 as a potential target for pro-senescence therapy in epithelial ovarian cancer. Cell Cycle 13:199–207
Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 136:62–74
Emadi Baygi M, Soheili ZS, Schmitz I, Sameie S, Schulz WA (2010) Snail regulates cell survival and inhibits cellular senescence in human metastatic prostate cancer cell lines. Cell Biol Toxicol 26:553–567
Minty F, Thurlow JK, Harrison PR, Parkinson EK (2008) Telomere dysfunction in human keratinocytes elicits senescence and a novel transcription profile. Exp Cell Res 314:2434–2447
Radiloff DR, Wakeman TP, Feng J, Schilling S, Seto E, Wang XF (2011) Trefoil factor 1 acts to suppress senescence induced by oncogene activation during the cellular transformation process. Proc Natl Acad Sci USA 108:6591–6596
Tran PT, Shroff EH, Burns TF, Thiyagarajan S, Das ST, Zabuawala T, Chen J, Cho YJ, Luong R, Tamayo P, Salih T, Aziz K, Adam SJ, Vicent S, Nielsen CH, Withofs N, Sweet-Cordero A, Gambhir SS, Rudin CM, Felsher DW (2012) Twist1 suppresses senescence programs and thereby accelerates and maintains mutant Kras-induced lung tumorigenesis. PLoS Genet 8:e1002650
Kwok WK, Ling MT, Yuen HF, Wong YC, Wang X (2007) Role of p14ARF in TWIST-mediated senescence in prostate epithelial cells. Carcinogenesis 28:2467–2475
Wang T, Li Y, Wang W, Tuerhanjiang A, Wu Z, Yang R, Yuan M, Ma D, Wang W, Wang S (2014) Twist2, the key Twist isoform related to prognosis, promotes invasion of cervical cancer by inducing epithelial-mesenchymal transition and blocking senescence. Hum Pathol 45:1839–1846
Han J, Kim YL, Lee KW, Her NG, Ha TK, Yoon S, Jeong SI, Lee JH, Kang MJ, Lee MG, Ryu BK, Baik JH, Chi SG (2013) ZNF313 is a novel cell cycle activator with an E3 ligase activity inhibiting cellular senescence by destabilizing p21(WAF1.). Cell Death Differ 20:1055–1067
Acknowledgements
We thank Joe Nassour for critical reading of the manuscript. This work was supported by the Centre National de la Recherche Scientifique, the Université Lille 1, the Université Lille 2, the Ligue contre le Cancer (Comité du Pas-de-Calais and Comité de la Somme), the Institut Pasteur de Lille, and the SIRIC OncoLille (Grant INCa-DGOS-Inserm 6041).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abbadie, C., Pluquet, O. & Pourtier, A. Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses?. Cell. Mol. Life Sci. 74, 4471–4509 (2017). https://doi.org/10.1007/s00018-017-2587-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2587-9