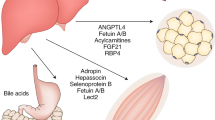
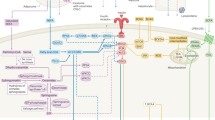
Abstract
Background
Organ crosstalk can be defined as the complex and mutual biological communication between distant organs mediated by signaling factors. Normally, crosstalk helps to coordinate and maintain homeostasis, but sudden or chronic dysfunction in any organ causes dysregulation in another organ. Many signal molecules, including cytokines and growth factors, are involved in the metabolic dysregulation, and excessive or inappropriate release of these molecules leads to organ dysfunction or disease (e.g., obesity, type 2 diabetes).
Aim and method
The aim of this review is to reveal the impact of organ crosstalk on the pathogenesis of diseases associated with organ interactions and the role of inflammatory and fibrotic changes in the organ dysfunction. After searching in MEDLINE, PubMed and Google Scholar databases using ‘organ crosstalk’ as a keyword, studies related to organ crosstalk and organ interaction were compiled and examined.
Conclusion
The organ crosstalk and the functional integration of organ systems are exceedingly complex processes. Organ crosstalk contributes to metabolic homeostasis and affects the inflammatory response, related pathways and fibrotic changes. As in the case of interactions between adipose tissue and intestine, stimulation of inflammatory mechanisms plays an active role in the development of diseases including insulin resistance, obesity, type 2 diabetes and hepatic steatosis. The increased level of knowledge about the ‘crosstalk’ between any organ and distant organs will facilitate the early diagnosis of the disease as well as the management of the treatment practices in the short- and long-term organ dysfunction.



Similar content being viewed by others
Abbreviations
- AGEs:
-
Advanced glycation end products
- AKI:
-
Acute kidney injury
- ALI:
-
Acute lung injury
- ANP:
-
Atrial natriuretic peptide
- AP-1:
-
Activator protein 1
- BNP:
-
B-type natriuretic peptide
- CKD:
-
Chronic kidney disease
- COX-2:
-
Cyclooxygenase-2
- CVD:
-
Cardiovascular disease
- CRP:
-
C-reactive protein
- DAMPs:
-
Damage-associated molecular patterns
- DM:
-
Diabetes mellitus
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial–mesenchymal transition
- ER:
-
Endoplasmic reticulum
- EVs:
-
Extracellular vesicles
- FFA:
-
Free fatty acids
- FGF:
-
Fibroblast growth factor
- FXR:
-
Farnesoid × receptor
- HFD:
-
High fat diet
- HIF-1:
-
Hypoxia-inducible factor 1
- IFN-γ:
-
Interferon gamma
- IR:
-
Insulin resistance
- IRI:
-
Ischemia/reperfusion injury
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- JNK:
-
c-Jun N-terminal kinase
- KIM-1:
-
Kidney injury molecule 1
- L-FABP:
-
Liver-type fatty acid-binding protein
- LPS:
-
Lipopolysaccharides
- MAPK:
-
Mitogen-activated protein kinase
- MYD88:
-
Myeloid differentiation factor 88
- NASH:
-
Non-alcoholic steatohepatitis
- NAFLD:
-
Non-alcoholic fatty liver disease
- NF-κB:
-
Nuclear factor kappa B
- NGAL:
-
Neutrophil gelatinase associated lipocalin
- NLRP3:
-
Nucleotide-binding domain 3
- NO:
-
Nitric oxide
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- NT-proBNP:
-
N-terminal pro–B-type natriuretic peptide
- MIF:
-
Macrophage migration inhibitory factor
- MODS:
-
Multiple organ dysfunction syndrome
- MOF:
-
Multiple organ failure
- PAI-1:
-
Plasminogen activator inhibitor-1
- PAMPs:
-
Pathogen-associated molecular patterns
- PNPLA3:
-
Patatin-like phospholipase domain-containing 3
- PI3:
-
Phosphatidylinositol 3-kinase
- PPAR-γ:
-
Peroxisome proliferator-activated receptor-γ
- ROS:
-
Reactive oxygen species
- SCFAs:
-
Short change fatty acids
- SIRS:
-
Systemic inflammatory response syndrome
- sST2:
-
Soluble suppressor of tumorigenicity 2
- STAT3:
-
Signal transducer and activator of transcription 3
- TECs:
-
Tubular epithelial cells
- TGF-β:
-
Transforming growth factor
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor alpha
- TNF-RII:
-
Tumor necrosis factor receptor II
References
Husain-Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, et al. Cardio-pulmonary-renal interactions: a multidisciplinary approach. J Am Coll Cardiol. 2015;65(22):2433–48.
Lane K, Dixon JJ, MacPhee IA, Philips BJ. Renohepatic crosstalk: does acute kidney injury cause liver dysfunction? Nephrol Dial Transplant. 2013;28:1634–47.
White LA, Chaudhary R, Moore LJ, Moore FA, Hassoun HT. Surgical sepsis and organ crosstalk: the role of the kidney. J Surg Res. 2011;167:306–15.
Kirpich IA, Marsano LS, McClain CJ. Gut–liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem. 2015;48(13–14):923–30.
Basu RK. Kidney–lung cross-talk and acute kidney injury. Pediatr Nephrol. 2013;28(12):2239–48.
Decleves AE, Sharma K. Obesity and kidney disease: differential effects of obesity on adipose tissue and kidney inflammation and fibrosis. Curr Opin Nephrol Hypertens. 2015;24(1):28–36.
Schmitz ML, Weber A, Roxlau T, Gaestel M, Kracht M. Signal integration, crosstalk mechanisms and networks in the function of inflammatory cytokines. Biochim Biophys Acta. 2011;1813(12):2165–75.
Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab. 2017;19(Suppl 1):137–46.
Roberts DD. Extracellular matrix and redox signaling in cellular responses to stress. Antioxid Redox Signal. 2017;27(12):771–3.
Poole LG, Dolin CE, Arteel GE. Organ–organ crosstalk and alcoholic liver disease. Biomolecules. 2017;7(3):E62. https://doi.org/10.3390/biom7030062.
Jahng JW, Song E, Sweeney G. Crosstalk between the heart and peripheral organs in heart failure. Exp Mol Med. 2016;48:e217. https://doi.org/10.1038/emm.2016.20.
Laurenzana I, Lamorte D, Trino S, De Luca L, Ambrosino C, Zoppoli P, et al. Extracellular vesicles: a new prospective in crosstalk between microenvironment and stem cells in hematological malignancies. Stem Cells Int. 2018;2018:9863194. https://doi.org/10.1155/2018/9863194.
Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. https://doi.org/10.1038/sigtrans.2017.23.
Zhao J, Lawless MW. Stop feeding cancer: pro-inflammatory role of visceral adiposity in liver cancer. Cytokine. 2013;64(3):626–37.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90.
Duvigneau JC, Luis A, Gorman AM, Samali A, Kaltenecker D, Moriggl R, et al. Crosstalk between inflammatory mediators and endoplasmic reticulum stress in liver diseases. Cytokine. 2018;1:2. https://doi.org/10.1016/j.cyto.2018.10.018.
Abais JM, Xia M, Zhang Y, Boini KM, Li P-L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111–29.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96.
Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. https://doi.org/10.1016/j.mam.2018.06.003.
Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1(1):21. https://doi.org/10.1186/2044-5040-1-21.
Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017;9(4):E387. https://doi.org/10.3390/nu9040387.
Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23(5):770–84.
Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. Non-alcoholic fatty liver and the gut microbiota. Mol Metab. 2016;5(9):782–94.
Zhang X, Ji X, Wang Q, Li JZ. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Protein Cell. 2018;9(2):164–77.
Tanabe K, Amo-Shiinoki K, Hatanaka M, Tanizawa Y. Interorgan crosstalk contributing to β-cell dysfunction. J Diabetes Res. 2017;2017:3605178. https://doi.org/10.1155/2017/3605178.
Ma M, Duan R, Zhong H, Liang T, Guo L. The crosstalk between fat homeostasis and liver regional immunity in NAFLD. J Immunol Res. 2019;2019:3954890.
Shirakawa J, De Jesus DF, Kulkarni RN. Exploring inter-organ crosstalk to uncover mechanisms that regulate β-cell function and mass. Eur J Clin Nutr. 2017;71(7):896–903.
Utzschneider KM, Kratz M, Damman CJ, Hullar M. Mechanisms linking the gut microbiome and glucose metabolism. J Clin Endocrinol Metab. 2016;101(4):1445–54.
Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–41.
Miura K, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139(1):323–34.
Patterson EK, Yao LJ, Ramic N, Lewis JF, Cepinskas G, McCaig L, et al. Lung-derived mediators induce cytokine production in downstream organs via an NF-κB dependent mechanism. Mediat Inflamm. 2013;2013:586895. https://doi.org/10.1155/2013/586895.
Fukazawa K, Lee HT. Updates on hepato-renal syndrome. J Anesth Clin Res. 2013;4(9):352.
Di Lullo L, Bellasi A, Barbera V, Russo D, Russo L, Di Iorio B, et al. Pathophysiology of the cardio-renal syndromes types 1–5: an uptodate. Indian Heart J. 2017;69(2):255–65.
Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018;19(1):285.
Lee SA, Cozzi M, Bush EL, Rabb H. Distant organ dysfunction in acute kidney injury: a review. Am J Kidney Dis. 2018;72(6):846–56.
Shiao CC, Wu PC, Huang TM, Lai TS, Yang WS, Wu CH, et al. Long-term remote organ consequences following acute kidney injury. Crit Care. 2015;19:438. https://doi.org/10.1186/s13054-015-1149-5.
Virzi GM, Day S, de Cal M, Vescovo G, Ronco C. Heart–kidney crosstalk and role of humoral signaling in critical illness. Crit Care. 2014;18:201.
White LE, Hassoun HT. Inflammatory mechanisms of organ crosstalk during ischemic acute kidney injury. Int J Nephrol. 2012;2012:505197. https://doi.org/10.4061/2012/505197.
Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200.
Trostel J, Garcia GE. Endogenous ınhibitors of kidney ınflammation. J Nephrol Res. 2015;1(2):61–8.
Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–96.
Zhou D, Liu Y. Renal fibrosis in 2015: understanding the mechanisms of kidney fibrosis. Nat Rev Nephrol. 2016;12:68–70.
Qi R, Yang C. Renal tubular epithelial cells: the neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018;9(11):1126.
Cantaluppi V, Quercia AD, Dellepiane S, Ferrario S, Camussi G, Biancone L. Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol Dial Transplant. 2014;29(11):2004–11.
Dellepiane S, Marengo M, Cantaluppi V. Detrimental cross-talk between sepsis and acute kidney injury: new pathogenic mechanisms, early biomarkers and targeted therapies. Crit Care. 2016;20:61. https://doi.org/10.1186/s13054-016-1219-3.
Kooman JP, Dekker MJ, Usvyat LA, Kotanko P, van der Sande FM, Schalkwijk CG, et al. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Renal Physiol. 2017;313(4):F938–50.
Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: the role of the kidney. Curr Opin Crit Care. 2009;15:481–7.
Domenech P, Perez T, Saldarini A, Uad P, Musso CG. Kidney-lung pathophysiological crosstalk: its characteristics and importance. Int Urol Nephrol. 2017;49(7):1211–5.
Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med. 2016;194(4):402–14.
Massey VL, Poole LG, Siow DL, Torres E, Warner NL, Schmidt RH, et al. Chronic alcohol exposure enhances lipopolysaccharide-induced lung injury in mice: potential role of systemic tumor necrosis factor alpha. Alcohol Clin Exp Res. 2015;39(10):1978–88.
Tashiro H, Shore SA. Obesity and severe asthma. Allergol Int. 2019;68(2):135–42.
Meiners S, Eickelberg O, Königshoff M. Hallmarks of the ageing lung. Eur Respir J. 2015;45:807–27.
Kathiriya JJ, Nakra N, Nixon J, Patel PS, Vaghasiya V, Alhassani A, et al. Galectin-1 inhibition attenuates profibrotic signaling in hypoxia-induced pulmonary fibrosis. Cell Death Discov. 2017;3:17010. https://doi.org/10.1038/cddiscovery.2017.10.
Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–91.
Schrier RW. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol. 2006;47(1):1–8.
Mitaka C, Si MK, Tulafu M, Yu Q, Uchida T, Abe S, et al. Effects of atrial natriuretic peptide on inter-organ crosstalk among the kidney, lung, and heart in a rat model of renal ischemia–reperfusion injury. Intensive Care Med Exp. 2014;2(1):28.
Mozaffari MS, Liu JY, Abebe W, Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis. 2013;3:180–96.
Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circ Res. 2012;110:126–44.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion, comprehensive. Physiology. 2016;7(1):113–70.
Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383(9932):1933–43.
Murtha LA, Schuliga MJ, Mabotuwana NS, et al. The processes and mechanisms of cardiac and pulmonary fibrosis. Front Physiol. 2017;8:777. https://doi.org/10.3389/fphys.2017.00777.
Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–69.
Piek A, de Boer RA, Sillje HH. The fibrosis-cell death axis in heart failure. Heart Fail Rev. 2016;21(2):199–211.
Bang C, Antoniades C, Antonopoulos AS, Eriksson U, Franssen C, Hamdani N, et al. Intercellular communication lessons in heart failure. Eur J Heart Fail. 2015;17(11):1091–103.
Cozzolino M, Ketteler M, Zehnder D. The vitamin D system: a crosstalk between the heart and kidney. Eur J Heart Fail. 2010;12(10):1031–41.
Romacho T, Elsen M, Röhrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf). 2014;210(4):733–53.
Hussain MA, Akalestou E, Song W-J. Inter-organ communication and regulation of beta cell function. Diabetologia. 2016;59:659–67.
Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37(4):1337–53.
Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–43.
Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2013;18:631–44.
van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–57.
Lakhani HV, Sharma D, Dodrill MW, Nawab A, Sharma N, Cottrill CL, et al. Phenotypic alteration of hepatocytes in non-alcoholic fatty liver disease. Int J Med Sci. 2018;14:1591–9.
Zhao HL, Sui Y, Guan J, et al. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int. 2008;74:467–77.
Zhu Q, Scherer PE. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat Rev Nephrol. 2018;14(2):105–20.
Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12(3):169–81.
Schroeder BO, Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22(10):1079–89.
Al Khodor S, Shatat I. Gut microbiome and kidney disease: a bidirectional relationship. Pediatr Nephrol. 2017;32(6):921–31.
Poeta M, Pierri L, Vajro P. Gut–liver axis derangement in non-alcoholic fatty liver disease. Children (Basel). 2017;4(8):E66. https://doi.org/10.3390/children4080066.
Bleau C, Karelis AD, St-Pierre DH, Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev. 2015;31(6):545–61.
Liu S, Alexander RK, Lee CH. Lipid metabolites as metabolic messengers in inter-organ communication. Trends Endocrinol Metab. 2014;25(7):356–63.
Cianci R, Pagliari D, Piccirillo CA, Fritz JH, Gambassi G. The microbiota and immune system crosstalk in health and disease. Mediators Inflamm. 2018;2018:2912539. https://doi.org/10.1155/2018/2912539 (eCollection 2018).
Everard A, Geurts L, Caesar R, Van Hul M, Matamoros S, Duparc T, et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun. 2014;5:5648. https://doi.org/10.1038/ncomms6648.
Jacobs R, Honore PM. Sepsis-induced multi-organ dysfunction syndrome—a mechanistic approach. J Emerg Crit Care Med. 2017;1:27. https://doi.org/10.21037/jeccm.2017.09.04.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72.
Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85.
Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67(3):483–98.
Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol. 2017;15(1):55–63.
Hamrick MW. Role of the cytokine-like hormone leptin in muscle-bone crosstalk with aging. J Bone Metab. 2017;24(1):1–8.
Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14(7):417–27.
Hatakeyama N, Matsuda N. Alert cell strategy: mechanisms of inflammatory response and organ protection. Curr Pharm Des. 2014;20(36):5766–78.
Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12(3):151–70.
John S, Willam C. Lung and kidney failure. Pathogenesis, interactions, and therapy. Med Klin Intensivmed Notfmed. 2015;110(6):452–8. https://doi.org/10.1007/s00063-014-0404-x.
Ologunde R, Zhao H, Lu K, Ma D. Organ cross talk and remote organ damage following acute kidney injury. Int Urol Nephrol. 2014;46(12):2337–45.
Ziesmann MT, Marshall JC. Multiple organ dysfunction: the defining syndrome of sepsis. Surg Infect. 2018;19(2):184–90.
Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14(4):217–30.
Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. https://doi.org/10.1038/nrdp.2016.45.
Fay KT, Ford ML, Coopersmith CM. The intestinal microenvironment in sepsis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10):2574–83. https://doi.org/10.1016/j.bbadis.2017.03.005.
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118(6):1021–40.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Chen D, Xie R, Shu B, Landay AL, Wei C, Reiser J, et al. Wnt signaling in bone, kidney, intestine, and adipose tissue and interorgan interaction in aging. Ann N Y Acad Sci. 2018;1442(1):48–60.
Biragyn A, Ferrucci L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018;19(6):e295–304.
Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54(Suppl):S29–37.
Toba H, Lindsey ML. Extracellular matrix roles in cardiorenal fibrosis: potential therapeutic targets for CVD and CKD in the elderly. Pharmacol Ther. 2019;193:99–120.
Panickar KS, Jewell DE. The benefit of anti-inflammatory and renal-protective dietary ingredients on the biological processes of aging in the kidney. Biology. 2018;7(4):E45. https://doi.org/10.3390/biology7040045.
Madero M, Katz R, Murphy R, Newman A, Patel K, Ix J, et al. Comparison between different measures of body fat with kidney function decline and incident CKD. Clin J Am Soc Nephrol. 2017;12(6):893–903.
Baskin KK, Winders BR, Olson EN. Muscle as a ‘‘mediator’’ of systemic metabolism. Cell Metab. 2015;21(2):237–48.
Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60(12):1031–42.
Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80(8):806–21.
Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: pGC-1α, myokines and exercise. Bone. 2015;80:115–25.
Peng H, Wang Q, Lou T, Qin J, Jung S, Shetty V, et al. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat Commun. 2017;8(1):1493.
Funding
There are no sources of funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Armutcu, F. Organ crosstalk: the potent roles of inflammation and fibrotic changes in the course of organ interactions. Inflamm. Res. 68, 825–839 (2019). https://doi.org/10.1007/s00011-019-01271-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01271-7