Abstract
Objective and Design
Inflammation is a key component of a number of diseases, including diabetic retinopathy. We investigated the cellular pathway by which protein kinase A (PKA) inhibited high mobility group box 1 (HMGB1).
Methods
Primary human retinal endothelial cells (REC) were grown in normal glucose (5 mM) or high glucose (25 mM). Cells in high glucose were treated with exchange protein for cAMP 1 (Epac1) and IGFBP-3 siRNA. Additional cells in high glucose were treated with forskolin, a PKA agonist, and Epac1 siRNA. Some cells were treated with a plasmid for insulin-like growth factor binding protein 3 (IGFBP-3) that does not bind IGF-1. Finally, some REC received Ex527, a sirtuin 1 (SIRT1) antagonist, prior to forskolin treatment. Protein analyses were done for HMGB1, Epac1, IGFBP-3, SIRT1, and PKA.
Results
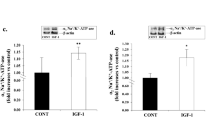
PKA inhibited cytoplasmic HMGB1, independent of Epac1 actions. PKA activated IGFBP-3 and SIRT1 to inhibit cytoplasmic HMGB1. High glucose inhibited SIRT1 levels and increased cytoplasmic HMGB1 in REC.
Conclusions
PKA requires active IGFBP-3 and SIRT1 to inhibit HMGB1 inflammatory actions in the retina vasculature. Activation of these pathways may offer new targets for therapy development.




Similar content being viewed by others
References
Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004; 18 1450–2.
Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress Retinal Eye Res 2011;30:343–58.
Abcouwer SF, Lin CM, Shanmugam S, et al. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflamm. 2013;10:149.
Zhang Q, Steinle JJ. IGFBP-3 inhibits TNF-alpha production and TNFR-2 signaling to protect against Retinal Endothelial Cell Apoptosis. Microvasc Res. 2014;95:76–81.
Zhang Q, Guy K, Pagadala J, et al. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 Levels. Investig Ophthalmol Vis Sci. 2012; 53 3004–13.
Jiang Y, Liu L, Curtiss E, Steinle JJ. Epac1 blocks NLRP3 inflammasome to reduce IL-1beta in retinal endothelial cells and mouse retinal vasculature. Mediators Inflamm. 2017; 2017 2860956.
Chen C, Du J, Feng W, et al. beta-Adrenergic receptors stimulate interleukin-6 production through Epac-dependent activation of PKCdelta/p38 MAPK signalling in neonatal mouse cardiac fibroblasts. Br J Pharmacol. 2012; 166:676–88.
Wu H, Chen Z, Xie J, et al. High mobility group box-1: a missing link between diabetes and its complications. Mediators Inflamm. 2016; 3896147.
Sun X, Zeng H, Wang Q, et al. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp Cell Res. 2018.
Abu El-Asrar AM, Siddiquei MM, Nawaz MI, Geboes K, Mohammad G. The proinflammatory cytokine high-mobility group box-1 mediates retinal neuropathy induced by diabetes. Mediators Inflamm. 2014; 2014 746415.
Dvoriantchikova G, Hernandez E, Grant J, et al. The high-mobility group box-1 nuclear factor mediates retinal injury after ischemia reperfusion. Investig Ophthalmol Vis Sci. 2011;52:7187–94.
Liu L, Jiang Y, Steinle JJ. Inhibition of HMGB1 protects the retina from ischemia-reperfusion, as well as reduces insulin resistance proteins. PloS One. 2017;12:e0178236.
Santos AR, Dvoriantchikova G, Li Y, et al. Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PloS One. 2014;9:e87574.
Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circul Res. 2007; 101:768–76.
Cho JH, Lee YK, Chae CB. The modulation of the biological activities of mitochondrial histone Abf2p by yeast PKA and its possible role in the regulation of mitochondrial DNA content during glucose repression. Biochimica et biophysica acta. 2001; 1522:175–86.
Ribeiro FS, de Abreu da Silva IC, Carneiro VC, et al. The dengue vector Aedes aegypti contains a functional high mobility group box 1 (HMGB1) protein with a unique regulatory C-terminus. PloS one. 2012;7:e40192.
Zhang Q, Steinle JJ. DNA-PK phosphorylation of IGFBP-3 is required to prevent apoptosis in retinal endothelial cells cultured in high glucose. Investig Ophthalmol Vis Sci. 2013;54:3052–7.
Rabadi MM, Xavier S, Vasko R, et al. High-mobility group box 1 is a novel deacetylation target of Sirtuin1. Kidney Int. 2015;87:95–108.
Nin V, Escande C, Chini CC, et al. Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase. J Biol Chem. 2012;287:23489–501.
Li J, Dou X, Li S, et al. Nicotinamide ameliorates palmitate-induced ER stress in hepatocytes via cAMP/PKA/CREB pathway-dependent Sirt1 upregulation. Biochimica et biophysica acta. 2015;1853:2929–36.
Choi YJ, Choi SE, Ha ES, et al. Extracellular visfatin activates gluconeogenesis in HepG2 cells through the classical PKA/CREB-dependent pathway. Hormone Metab. 2014; 46 233–9.
Zhang Q, Jiang Y, Miller MJ, et al. IGFBP-3 and TNF-alpha regulate retinal endothelial cell apoptosis. Investig Ophthalmol Vis Sci. 2013;54:5376–84.
Mohammad G, Alam K, Nawaz MI, et al. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J Physiol Biochem. 2015; 71:359–72.
Ha YM, Ham SA, Kim YM, et al. beta(1)-adrenergic receptor-mediated HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW 264.7 cells leads to inhibition of HMGB1 release in LPS-activated RAW 264.7 cells and increases in survival rate of CLP-induced septic mice. Biochem Pharmacol. 2011; 82:769–77.
Liu L, Jiang Y, Chahine A, Curtiss E, Steinle JJ. Epac1 agonist decreased inflammatory proteins in retinal endothelial cells, and loss of Epac1 increased inflammatory proteins in the retinal vasculature of mice. Molecular Vision. 2017;23:1–7.
Takahashi H, Sadamori H, Teshigawara K, et al. Histamine inhibits high mobility group box 1-induced adhesion molecule expression on human monocytes. Eur J Pharmacol. 2013; 718:305–13.
Zuo Z, Che X, Wang Y, et al. High mobility group Box-1 inhibits cancer cell motility and metastasis by suppressing activation of transcription factor CREB and nWASP expression. Oncotarget. 2014;5:7458–70.
Hu P, Thinschmidt JS, Caballero S, et al. Loss of survival factors and activation of inflammatory cascades in brain sympathetic centers in type 1 diabetic mice. Am J Physiol Endocrinol Metab. 2015;308:E688-98.
Thinschmidt JS, Colon-Perez LM, Febo M, et al. Depressed basal hypothalamic neuronal activity in type-1 diabetic mice is correlated with proinflammatory secretion of HMBG1. Neurosci Lett. 2016; 615:21–7.
Wang Y, Zhao X, Shi D, et al. Overexpression of SIRT1 promotes high glucose-attenuated corneal epithelial wound healing via p53 regulation of the IGFBP3/IGF-1R/AKT pathway. Investigative ophthalmology & visual science. 2013; 54 3806–14.
Acknowledgements
R01EY028442 (JJS), P30EY04068 (Hazlett), and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute). The funders did not influence these design or execution of these studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Liu, L., Patel, P. & Steinle, J.J. PKA regulates HMGB1 through activation of IGFBP-3 and SIRT1 in human retinal endothelial cells cultured in high glucose. Inflamm. Res. 67, 1013–1019 (2018). https://doi.org/10.1007/s00011-018-1196-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1196-x