Abstract
Objective and design
MicroRNAs (miRNAs) play an important role in the pathogenesis of human diseases by regulating the expression of target genes in specific cells or tissues. In this study, we analyzed the association between the MIR429 and its target gene, charged multivesicular body protein 5 (CHMP5), in human colon cancer cells and in a DSS-induced colitis mouse model.
Materials and methods
A luciferase reporter system was used to confirm the effect of MIR429 on CHMP5 expression. Protein or mRNA expression of the target gene and associated molecules were measured by Western blot or quantitative RT-PCR (qRT-PCR), respectively. Flow cytometry was used to compare cell viability or cell cycle progression.
Results
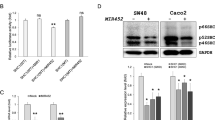
CHMP5 mRNA and protein expression was directly down-regulated by MIR429. We found that MIR429 inhibited colon cancer cell growth and cell cycle progression, and CHMP5 was overexpressed in the DSS-induced colitis mouse model and human ulcerative colitis (UC) tissues.
Conclusions
Our findings show that CHMP5 is a direct target of MIR429 in human colon cancer cell lines and suggest that CHMP5 up-regulation as a result of reduced MIR429 expression in DSS-induced mice colitis tissues and human UC tissues may restrict apoptosis and promote cell proliferation.





Similar content being viewed by others
References
Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205.
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29.
Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648–56.
Travis S, Jewell DP. Ulcerative colitis: clinical presentation and diagnosis. In: Satsangi J, Sutherland LR, editors. Inflammatory bowel disease. New York: Churchill Livingstone; 2003. pp. 169–82.
Forbes A. Clinical presentation and diagnosis of Crohn’s disease. In: Satsangi J, Sutherland L, editors. Inflammatory bowel disease. New York: Churchill Livingstone; 2003. pp. 183–9.
Pekow JR, Kwon JH. MicroRNAs in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:187–93.
Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5.
Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20.
Sun T, Wang C, Xing J, Wu D. MiR-429 modulates the expression of c-myc inhuman gastric carcinoma cells. Eur J Cancer. 2011;47:2552–9.
Li J, Du L, Yang Y, Wang C, Liu H, Wang L, et al. MiR-429 is an independent prognostic factor in colorectal cancer an dexerts its anti-apoptotic function by targeting SOX2. Cancer Lett. 2013;329:84–90.
Mo JS, Alam KJ, Kim HS, Lee YM, Yun KJ, Chae SC. MicroRNA 429 regulates mucin gene expression and secretion in murine model of colitis. J Crohns Colitis. 2016;10:837–49.
Mo JS, Alam KJ, Kang IH, Park WC, Seo GS, Choi SC, et al. MicroRNA 196B regulates FAS-mediated apoptosis in colorectal cancer cells. Oncotarget. 2015;6:2843–55.
Alam KJ, Mo JS, Han SH, Park WC, Kim HS, Yun KJ, Chae SC. MicroRNA 375 regulates proliferation and migration of colon cancer cells by suppressing the CTGF-EGFR signaling pathway. Int J Cancer. 2017;141:1614–29.
Ahmed D, Eide PW, Eilertsen IA, Vaiopoulou A, Nikiteas N. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71.
Archanioti P, Gazouli M, Theodoropoulos G, Vaiopoulou A, Nikiteas N. Micro-RNAs as regulators and possible diagnostic bio-markers in inflammatory bowel disease. J Crohns Colitis. 2011;5:520–4.
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34.
Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterol. 1995;109:1344–67.
Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905.
Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003;15:446–55.
Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–52.
Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–9.
Shim JH, Xiao C, Hayden MS, Lee KY, Trombetta ES, Pypaert M, et al. CHMP5 is essential for late endosome function and downregulation of receptor signaling during mouse embryogenesis. J Cell Biol. 2006;172:1045–56.
Wang HR, Gu CH, Zhu JY, Han JY, Zhong H, Chen FY, Ouyang RR. PNAS-2: a novel gene probably participating in leukemogenesis. Oncology. 2006;71:423–9.
Shahmoradgoli M, Mannherz O, Engel F, Han JY, Zhong H, Chen FY, Ouyang RR. Antiapoptotic function of charged multivesicular body protein 5: a potentially relevant gene in acute myeloid leukemia. Int J Cancer. 2011;128:2865–71.
Thiebaut R, Esmiol S, Lecine P, Mahfouz B, Hermant A, Nicoletti C, et al. Characterization and genetic analyses of new genes coding for NOD2 interacting proteins. PLoS One. 2016;11:e0165420.
Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40.
Acknowledgements
The biospecimens for this study were provided by the Biobank of Wonkwang University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (2017R1A2B4004801).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical standards
This research was conducted with the approval of the institutional review board (IRB) of Wonkwang University.
Additional information
Responsible Editor: John Di Battista.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11_2018_1194_MOESM1_ESM.tif
Fig. S1 The immunohistochemistry results of LCA and CHMP5 in DSS-induced mice colitis and human UC colon tissue. (A) H&E staining of DSS-induced colitis colon and normal colon consecutive tissue. Inflammation was observed in DSS-induced colitis colon tissue (single head arrow). LCA and CHMP5 expression levels in DSS-induced colitis colons (double head arrow) are higher than that of normal colons (single head arrow). (B) The immunohistochemistry results of CHMP5 in six human UC colon tissues and six normal colon tissues (×400). The CHMP5 expression levels are significantly increased by the severity of inflammation in the lamina propria of human colon (TIF 750 KB)
Rights and permissions
About this article
Cite this article
Mo, JS., Han, SH., Yun, KJ. et al. MicroRNA 429 regulates the expression of CHMP5 in the inflammatory colitis and colorectal cancer cells. Inflamm. Res. 67, 985–996 (2018). https://doi.org/10.1007/s00011-018-1194-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1194-z