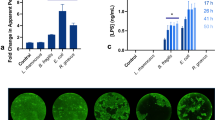
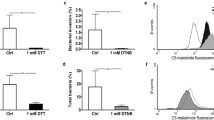
Abstract
Uncoupling of oxidative phosphorylation in epithelial mitochondria results in decreased epithelial barrier function as characterized by increased internalization of non-invasive Escherichia coli and their translocation across the epithelium. We hypothesized that the increased burden of intracellular commensal bacteria would activate the enterocyte, with the potential to promote inflammation. Treatment of human colon-derived epithelial cell lines in vitro with dinitrophenol (DNP) and commensal E. coli (strains F18, HB101) provoked increased production of interleukin (IL-8), which was not observed with conditioned medium from the bacteria, lipopolysaccharide or inert beads. The IL-8 response was inhibited by co-treatment with cytochalasin-D (blocks F-actin rearrangement), chloroquine (blocks phagosome acidification) and a MyD88 inhibitor (blocks TLR signaling), consistent with TLR-signaling mediating IL-8 synthesis subsequent to bacterial internalization. Use of the mitochondria-targeted antioxidant, mitoTEMPO, or U0126 to block ERK1/2 MAPK signalling inhibited DNP+E. coli-evoked IL-8 production. Mutations in the NOD2 (the intracellular sensor of bacteria) or ATG16L1 (autophagy protein) genes are susceptibility traits for Crohn’s, and epithelia lacking either protein displayed enhanced IL-8 production in comparison to wild-type cells when exposed to DNP + E coli. Thus, metabolic stress perturbs the normal epithelial–bacterial interaction resulting in increased IL-8 production due to uptake of bacteria into the enterocyte: this potentially pro-inflammatory event is enhanced in cells lacking NOD2 or ATG16L1 that favor increased survival of bacteria within the enterocyte. We speculate that by increasing epithelial permeability and IL-8 production, reduced mitochondria function in the enteric epithelium would contribute to the initiation, pathophysiology, and reactivation of inflammatory disease in the gut.







Similar content being viewed by others
References
Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21.
Baggiolini M. CXCL8 - The First Chemokine. Front Immunol. 2015;6:285.
Balestrieri ML, Balestrieri A, Mancini FP, Napoli C. Understanding the immunoangiostatic CXC chemokine network. Cardiovasc Res. 2008;78:250–6.
Russo RC, Garcia CC, Teixeira MM. Anti-inflammatory drug development: broad or specific chemokine receptor antagonists? Curr Opin Drug Discov Devel. 2010;13:414–27.
Schuller S, Lucas M, Kaper JB, Giron JA, Phillips AD. The ex vivo response of human intestinal mucosa to enteropathogenic Escherichia coli infection. Cellul Micro. 2009;11:521–30.
Kvedaraite E, Lourda M, Idestrom M, Chen P, Olsson-Akefeldt S, Forkel M, et al. Tissue-infiltrating neutrophils represent the main source of IL-23 in the colon of patients with IBD. Gut. 2016;65:1632–41.
Song F, Ito K, Denning TL, Kuninger D, Papaconstantinou J, Gourley W, et al. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J Immunol. 1999;162:2275–80.
Schoultz I, Soderholm JD, McKay DM. Is metabolic stress a common denominator in inflammatory bowel disease? Inflamm Bowel Dis. 2011;17:2008–18.
Rath E, Haller D. Mitochondria at the interface between danger signaling and metabolism: role of unfolded protein responses in chronic inflammation. Inflamm Bowel Dis. 2012;18:1364–77.
Nazli A, Yang PC, Jury J, Howe K, Watson JL, Soderholm JD, et al. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol. 2004;164:947–57.
Fougeray S, Bouvier N, Beaune P, Legendre C, Anglicheau D, Thervet E, et al. Metabolic stress promotes renal tubular inflammation by triggering the unfolded protein response. Cell Death Dis. 2011;2:e143.
Saxena A, Lopes F, Poon KKH, McKay DM. Absence of the NOD2 protein renders epithelia more susceptible to barrier dysfunction due to mitochondrial dysfunction. Am J Physiol Gastrointest Liver Physiol. 2017;313:G26–38.
Lopes F, Keita AV, Saxena A, Reyes JL, Mancini NL, Al Rajabi A, et al. ER-stress mobilization of death-associated protein kinase-1-dependent xenophagy counteracts mitochondria stress-induced epithelial barrier dysfunction. J Biol Chem. 2018;293:3073–87.
Nazli A, Wang A, Steen O, Prescott D, Lu J, Perdue MH, et al. Enterocyte cytoskeleton changes are crucial for enhanced translocation of nonpathogenic Escherichia coli across metabolically stressed gut epithelia. Infect Immun. 2006;74:192–201.
Smyth D, McKay CM, Gulbransen BD, Phan VC, Wang A, McKay DM. Interferon-g signals via an ERK1/2-ARF6 pathway to promote bacterial internalization by gut epithelia. Cell Microbiol. 2012;14:1257–70.
Wang A, Keita AV, Phan V, McKay CM, Schoultz I, Lee J, et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Path. 2014;184:2516–27.
Fernando MR, Saxena A, Reyes JL, McKay DM. Butyrate enhances antibacterial effects while suppressing other features of alternative activation in IL-4-induced macrophages. Am J Physiol Gastrointest Liver Physiol. 2016;310:G822-31.
Fernando EH, Dicay M, Stahl M, Gordon MH, Vegso A, Baggio C, et al. A simple, cost-effective method for generating murine colonic 3D enteroids and 2D monolayers for studies of primary epithelial cell function. Am J Physiol Gastrointest Liver Physiol. 2017;313:G467–75.
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5.
Ewaschuk JB, Backer JL, Churchill TA, Obermeier F, Krause DO, Madsen KL. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun. 2007;75:2572–9.
Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infection. 2012;18(Suppl 4):2–4.
Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–64.
Banan A, Fields JZ, Farhadi A, Talmage DA, Zhang L, Keshavarzian A. Activation of delta-isoform of protein kinase C is required for oxidant-induced disruption of both the microtubule cytoskeleton and permeability barrier of intestinal epithelia. J Pharmacol Exp Ther. 2002;303:17–28.
Kvasnovsky CL, Aujla U, Bjarnason I. Nonsteroidal anti-inflammatory drugs and exacerbations of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:255–63.
Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcytosis of commensal bacteria via lip rafts. Gut Pathog. 2009;1:2.
McKay DM, Watson JL, Wang A, Caldwell J, Prescott D, Ceponis PM, et al. Phosphatidylinositol 3′-kinase is a critical mediator of interferon-gamma-induced increases in enteric epithelial permeability. J Pharm Exp Therap. 2007;320:1013–22.
Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon-g induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258–67.
Kubes P. The enigmatic neutrophil: what we do not know. Cell Tiss Res. 2018;371:399–406.
Zheng J, Meng J, Zhao S, Singh R, Song W. Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-kB. Infect Immun. 2008;76:4498–508.
Gewirtz AT, Rao AS, Simon PO Jr, Merlin D, Carnes D, Madara JL, et al. Salmonella typhimurium induces epithelial IL-8 expression via Ca2+-mediated activation of the NF-kB pathway. J Clin Invest. 2000;105:79–92.
Crowe SE, Alvarez L, Dytoc M, Hunt RH, Muller M, Sherman PM, Patel J, Jin Y, Ernst PB. Expression of interleukin-8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;109:65–74.
Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47:79–87.
Ohkusa T, Yoshida T, Sato N, Watanabe S, Tajiri H, Okayasu I. Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: a possible pathogenic mechanism of ulcerative colitis. J Med Microbiol. 2009;58:535–45.
Bahrami B, Macfarlane S, Macfarlane GT. Induction of cytokine formation by human intestinal bacteria in gut epithelial cell lines. J Appl Microbiol. 2011;110:353–63.
Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kB. Inter J Mol Med. 1999;4:223–30.
Bernhart E, Kogelnik N, Prasch J, Gottschalk B, Goeritzer M, Depaoli MR, et al. 2-Chlorohexadecanoic acid induces ER stress and mitochondrial dysfunction in brain microvascular endothelial cells. Redox Biol. 2018;15:441–51.
Guo Z, Hong Z, Dong W, Deng C, Zhao R, Xu J, et al. PM2.5-induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. Inter J Environ Res Pub Health. 2017; 14.
Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–60.
Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kB-independent pathway. FASEB J. 2003;17:1319–21.
Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014;40:843–54.
Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72.
Homer CR, Richmond AL, Rebert NA, Achkar JP, McDonald C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology. 2010;139:1630–41.
Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445–59.
Irving AT, Mimuro H, Kufer TA, Lo C, Wheeler R, Turner LJ, et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe. 2014;15:623–35.
Salem M, Ammitzboell M, Nys K, Seidelin JB, Nielsen OH. ATG16L1: a multifunctional susceptibility factor in Crohn disease. Autophagy. 2015;11:585–94.
Murthy A, Li Y, Peng I, Reichelt M, Katakam AK, Noubade R, et al. A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014;506:456–62.
Lassen KG, Kuballa P, Conway KL, Patel KK, Becker CE, Peloquin JM, et al. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Nat Acad Sci (USA). 2014;111:7741–6.
Acknowledgements
A. Saxena was supported by a Queen Elizabeth II Graduate Studentship Award. F. Lopes was supported by CIHR/Canadian Association of Gastroenterology (CAG)/Allergan Inc. and Alberta Innovates-Health solutions (AI-HS) Post-Doctoral Fellowships. D.M. McKay holds a Canada Research Chair (CRC: Tier 1) in Intestinal Immunophysiology in Health and Disease.
Funding
Funding provided by a Canadian Institutes for Health Research (CIHR) Operating Grant (MPO-126005) to D.M. McKay.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Saxena, A., Lopes, F. & McKay, D.M. Reduced intestinal epithelial mitochondrial function enhances in vitro interleukin-8 production in response to commensal Escherichia coli. Inflamm. Res. 67, 829–837 (2018). https://doi.org/10.1007/s00011-018-1172-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1172-5