Abstract
Objective
The peptide lycosin-I has anti-bacterial and anti-cancer capacities. However, the anti-inflammatory activity of lycosin-I remains unknown. We investigated whether lycosin-I could attenuate inflammation.
Materials and methods
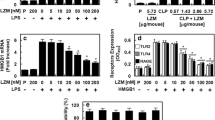
Human umbilical vein endothelial cells (HUVECs) were treated with lycosin-I before exposure to tumor necrosis factor-α (TNF-α). The expression of intercellular cell adhesion molecule-1 (ICAM-1), nuclear transcription factor-kappa B (NF-κB) p65 and inhibitory subunit of NF-κB alpha (IκBα) was evaluated by western blot. The expression of interleukin-6 (IL-6) and interleukin-8 (IL-8) was detected by quantitative RT-PCR or ELISA. Immunofluorescence analysis was used to determine the impact of lycosin-I on NF-κB pathway. C57BL/6 mice were pretreated with lycosin-I before exposure with lipopolysaccharide (LPS).
Results
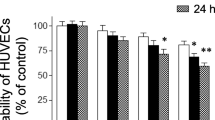
Lycosin-I significantly reduced the TNF-α-enhanced expression of IL-6, IL-8 and ICAM-1. Lycosin-I also inhibited the human monocyte cells adhesion to HUVECs. We further demonstrated that lycosin-I could effectively suppress the reaction of endothelial cells to TNF-α by inhibiting IκBα degradation. Subsequently, the phosphorylation and translocation of NF-κB p65 could also be attenuated. Furthermore, lycosin-I exhibited a significant protection of C57BL/6 mice against LPS-induced death.
Conclusions
Our results suggested that the anti-inflammatory activity of lycosin-I was associated with NF-κB activation and lycosin-I had potential to be a novel therapeutic candidate for inflammatory diseases.








Similar content being viewed by others
References
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115 – 26. https://doi.org/10.1056/nejm199901143400207.
Manduteanu I, Simionescu M. Inflammation in atherosclerosis: a cause or a result of vascular disorders? J Cell Mol Med. 2012;16(9):1978–90. https://doi.org/10.1111/j.1582-4934.2012.01552.x.
Libby P. Inflammation in atherosclerosis. arteriosclerosis, thrombosis, and vascular biology. 2012;32(9):2045–51. https://doi.org/10.1161/atvbaha.108.179705.
Yu BL, Wang SH, Peng DQ, Zhao SP. HDL and immunomodulation: an emerging role of HDL against atherosclerosis. Immunol Cell Biol. 2010;88(3):285–90. https://doi.org/10.1038/icb.2009.112.
Kinlay S, Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol. 1997;80(9A):11I-6I.
Old LJ. Tumor necrosis factor (TNF). Science. 1985;230(4726):630–2.
Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115(1):1–20. https://doi.org/10.1111/j.1365-2567.2005.02143.x.
Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12(2):147–68. https://doi.org/10.1038/nrd3930.
Chai H, Wang Q, Huang L, Xie T, Fu Y. Ginsenoside Rb1 inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Biol Pharm Bull. 2008;31(11):2050–6.
Chang CC, Chu CF, Wang CN, Wu HT, Bi KW, Pang JH, et al. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-alpha-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomed: Int J Phytother Phytopharmacol. 2014;21(3):207 – 16. https://doi.org/10.1016/j.phymed.2013.09.012.
Sengul S, Zwizinski C, Batuman V. Role of MAPK pathways in light chain-induced cytokine production in human proximal tubule cells. Am J Physiol Renal Physiol. 2003;284(6):F1245-54. https://doi.org/10.1152/ajprenal.00350.2002.
de Haij S, Bakker AC, van der Geest RN, Haegeman G, Vanden Berghe W, Aarbiou J, et al. NF-kappaB mediated IL-6 production by renal epithelial cells is regulated by c-jun NH2-terminal kinase. J Am Soc Nephrol: JASN. 2005;16(6):1603–11. https://doi.org/10.1681/asn.2004090781.
Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Investig. 1998;101(2):353–63. https://doi.org/10.1172/jci1195.
Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398(6729):718–23. https://doi.org/10.1038/19546.
Fenyo IM, Gafencu AV. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology. 2013;218(11):1376–84. https://doi.org/10.1016/j.imbio.2013.06.005.
Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–21. https://doi.org/10.1038/nri3520.
Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Ann Rev Immunol. 1994;12:141–79. https://doi.org/10.1146/annurev.iy.12.040194.001041.
Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9(10):899–909.
Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harbor Perspect Biol. 2012;4(3). https://doi.org/10.1101/cshperspect.a006049.
Morris-Rosenfeld S, Lipinski MJ, McNamara CA. Understanding the role of B cells in atherosclerosis: potential clinical implications. Expert Rev Clin Immunol. 2014;10(1):77–89. https://doi.org/10.1586/1744666x.2014.857602.
Kondkar AA, Abu-Amero KK. Utility of circulating microRNAs as clinical biomarkers for cardiovascular diseases. BioMed Res Int. 2015;2015:821823. https://doi.org/10.1155/2015/821823.
Lee SW, Kim HC, Lee HS, Suh I. Erratum to: thirty-year trends in mortality from cardiovascular diseases in Korea. Korean Circ J. 2015;45(5):442. https://doi.org/10.4070/kcj.2015.45.5.442.
Abubakar II, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. https://doi.org/10.1016/s0140-6736(14)61682-2.
Skarnes RC, Watson DW. Antimicrobial factors of normal tissues and fluids. Bacteriol Rev. 1957;21(4):273–94.
Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochimica et biophysica acta. 1999;1462(1–2):11–28.
Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–50. https://doi.org/10.1038/nrmicro1098.
Wang L, Wang YJ, Liu YY, Li H, Guo LX, Liu ZH, et al. In vitro potential of Lycosin-I as an alternative antimicrobial drug for treatment of multidrug-resistant Acinetobacter baumannii infections. Antimicrobial Agents Chemother. 2014;58(11):6999–7002. https://doi.org/10.1128/AAC.03279-14.
Huang L, Tang Y, Qin J, Peng Y, Yuan Q, Zhang F, et al. Vasoactive intestinal peptide enhances TNF-alpha-induced IL-6 and IL-8 synthesis in human proximal renal tubular epithelial cells by NF-kappaB-dependent mechanism. Inflammation. 2012;35(3):1154–60. https://doi.org/10.1007/s10753-011-9423-4.
Zhang Z, Mu L, Tang J, Duan Z, Wang F, Wei L, et al. A small peptide with therapeutic potential for inflammatory acne vulgaris. PLoS One. 2013;8(8):e72923. https://doi.org/10.1371/journal.pone.0072923.
Fontes JA, Rose NR, Cihakova D. The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine. 2015;74(1):62–8. https://doi.org/10.1016/j.cyto.2014.12.024.
Feng M, Shu Y, Yang Y, Zheng X, Li R, Wang Y, et al. Ulinastatin attenuates experimental autoimmune encephalomyelitis by enhancing anti-inflammatory responses. Neurochem Int. 2014;64:64–72. https://doi.org/10.1016/j.neuint.2013.11.007.
Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circulation research. 2007;101(3):234–47. https://doi.org/10.1161/CIRCRESAHA.107.151860b.
Wang B, Wei H, Prabhu L, Zhao W, Martin M, Hartley AV, et al. Role of novel serine 316 phosphorylation of the p65 subunit of NF-kappaB in differential gene regulation. J Biol Chem. 2015;290(33):20336–47. https://doi.org/10.1074/jbc.M115.639849.
Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30(1):43–52. https://doi.org/10.1016/j.tibs.2004.11.009.
Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–71. https://doi.org/10.1056/nejm199704103361506.
Brown JD, Lin CY, Duan Q, Griffin G, Federation AJ, Paranal RM, et al. NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56(2):219–31. https://doi.org/10.1016/j.molcel.2014.08.024.
Mako V, Czucz J, Weiszhar Z, Herczenik E, Matko J, Prohaszka Z, et al. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1beta, TNF-alpha, and LPS. Cytometry Part A J Int Soc Anal Cytol. 2010;77(10):962–70. https://doi.org/10.1002/cyto.a.20952.
Chistiakov DA, Sobenin IA, Orekhov AN. Regulatory T cells in atherosclerosis and strategies to induce the endogenous atheroprotective immune response. Immunol Lett. 2013;151(1–2):10–22. https://doi.org/10.1016/j.imlet.2013.01.014.
Coumbe AG, Pritzker MR, Duprez DA. Cardiovascular risk and psoriasis: beyond the traditional risk factors. Am J Med. 2014;127(1):12–8. https://doi.org/10.1016/j.amjmed.2013.08.013.
Khalil RA. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol. 2013;86(12):1627–42. https://doi.org/10.1016/j.bcp.2013.09.024.
Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–34. https://doi.org/10.1038/nri910.
Fu Y, Wei Z, Zhou E, Zhang N, Yang Z. Cyanidin-3-O-beta-glucoside inhibits lipopolysaccharide-induced inflammatory response in mouse mastitis model. J Lipid Res. 2014;55(6):1111–9. https://doi.org/10.1194/jlr.M047340.
Ding Y, Liao W, He X, Xiang W, Lu Q. CSTMP exerts anti-inflammatory effects on LPS-induced human renal proximal tubular epithelial cells by inhibiting TLR4-mediated NF-kappaB pathways. Inflammation. 2016;39(2):849–59. https://doi.org/10.1007/s10753-016-0315-5.
Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995;270(2):933–43.
Lockyer JM, Colladay JS, Alperin-Lea WL, Hammond T, Buda AJ. Inhibition of nuclear factor-kappaB-mediated adhesion molecule expression in human endothelial cells. Circul Res. 1998;82(3):314–20.
Wang L, Xu Y, Yu Q, Sun Q, Gu Q, Xu X. H-RN, a novel antiangiogenic peptide derived from hepatocyte growth factor inhibits inflammation in vitro and in vivo through PI3K/AKT/IKK/NF-kappaB signal pathway. Biochem Pharmacol. 2014;89(2):255–65. https://doi.org/10.1016/j.bcp.2014.02.026.
Wan M, Liu J, Ouyang X. Nucleotide-binding oligomerization domain 1 regulates Porphyromonas gingivalis-induced vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 expression in endothelial cells through NF-kappaB pathway. J Periodontal Res. 2015;50(2):189–96. https://doi.org/10.1111/jre.12192.
Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Investig. 2001;107(3):255–64. https://doi.org/10.1172/jci10373.
Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86(2):211–8. https://doi.org/10.1093/cvr/cvq076.
Kurokouchi K, Kambe F, Kikumori T, Sakai T, Sarkar D, Ishiguro N, et al. Effects of glucocorticoids on tumor necrosis factor alpha-dependent activation of nuclear factor kappaB and expression of the intercellular adhesion molecule 1 gene in osteoblast-like ROS17/2.8 cells. J Bone Miner Res. 2000;15(9):1707–15. https://doi.org/10.1359/jbmr.2000.15.9.1707.
Eberhardt W, Schulze M, Engels C, Klasmeier E, Pfeilschifter J. Glucocorticoid-mediated suppression of cytokine-induced matrix metalloproteinase-9 expression in rat mesangial cells: involvement of nuclear factor-kappaB and Ets transcription factors. Mol Endocrinol (Baltimore. Md). 2002;16(8):1752–66. https://doi.org/10.1210/me.2001-0278.
Vital AL, Goncalo M, Cruz MT, Figueiredo A, Duarte CB, Lopes MC. Dexamethasone prevents granulocyte-macrophage colony-stimulating factor-induced nuclear factor-kappaB activation, inducible nitric oxide synthase expression and nitric oxide production in a skin dendritic cell line. Mediators Inflammation. 2003;12(2):71–8. https://doi.org/10.1080/0962935031000097673.
Ma W, Gee K, Lim W, Chambers K, Angel JB, Kozlowski M, et al. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-kappa B transcription factors. J Immunol (Baltimore: 1950). 2004;172(1):318–30.
Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, et al. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol (Baltimore: 1950). 2004;172(10):6336–44.
Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–11. https://doi.org/10.1056/NEJMoa1500896.
Luan YY, Dong N, Xie M, Xiao XZ, Yao YM. The significance and regulatory mechanisms of innate immune cells in the development of sepsis. J Interferon Cytokine Res. 2014;34(1):2–15. https://doi.org/10.1089/jir.2013.0042.
Luan YY, Yao YM, Xiao XZ, Sheng ZY. Insights into the apoptotic death of immune cells in sepsis. J Interferon Cytokine Res. 2015;35(1):17–22. https://doi.org/10.1089/jir.2014.0069.
Acknowledgements
This project was supported by the Natural Science Foundation of Hunan Province (2015JJ2192 and 2017JJ2372), China Postdoctoral Science Foundation (2015M580704), Scientific Research Foundation of Central South University (2014JSJJ027), the National Natural Science Foundation of China (30901874 and 31672290) and National Undergraduate Innovation Training Program of Central South University (201510533321 and 201610533535). The authors are grateful to Dr. Songping, Liang and Zhonghua Liu, Hunan Normal University, for providing professional advice.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by Bernhard Gibbs.
Rights and permissions
About this article
Cite this article
Li, X., Tang, Y., Ma, B. et al. The peptide lycosin-I attenuates TNF-α-induced inflammation in human umbilical vein endothelial cells via IκB/NF-κB signaling pathway. Inflamm. Res. 67, 455–466 (2018). https://doi.org/10.1007/s00011-018-1138-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1138-7