Abstract
Background and aim
Studies have verified the protective effect of Hydrogen Sulfide (H2S) on gastric ulcer and ulcerative colitis, but the mechanisms are not fully illustrated. In this study, the possible protective effect of H2S on TNF-α/IFN-γ induced barrier dysfunction was investigated in Caco-2 cell monolayers.
Method
The barrier function of Caco-2 monolayers was evaluated by measuring trans-epithelial electrical resistance (TEER) and FITC-Dextran 4 kDa (FD-4) trans-membrane flux. ZO-1 and Occludin were chosen as markers of the localization of tight junction (TJ) proteins for immunofluorescence. The expression of MLCK and phosphorylation level of myosin light chain (MLC) were measured by immunoblotting. The activation of NF-kB p65 was analyzed by EMSA and immunofluorescence.
Results
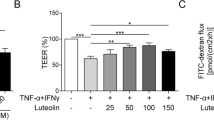
NaHS at 500uM significantly attenuated TNF-α/IFN-γ-indueced Caco-2 monolayer barrier injury. The increased expression of MLCK and increased phosphorylation level of MLC induced by TNF-α/IFN-γ was also inhibited significantly by NaHS. Additionally, NaHS inhibited TNF-α/IFN-γ induced activation and nuclear translocation of NF-kB p65.
Conclusion
The present study reveals the protective effect of H2S on TNF-α and IFN-γ-induced injury of intestinal epithelial barrier function in Caco-2 monolayers and suggests that the suppression of MLCK-P-MLC signaling mediated by NF-kB P65 might be one of the mechanisms underlying the protective effect of H2S.






Similar content being viewed by others
References
Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809.
Lin N, Xu LF, Sun M. The protective effect of trefoil factor 3 on the intestinal tight junction barrier is mediated by toll-like receptor 2 via a PI3 K/Akt dependent mechanism. Biochem Biophys Res Commun. 2013;440(1):143–9.
Chen SW, Wang PY, Zhu J, Chen GW, Zhang JL, Chen ZY, Zuo S, Liu YC, Pan YS. Protective effect of 1,25-Dihydroxyvitamin D3 on lipopolysaccharide-induced intestinal epithelial tight Junction injury in Caco-2 cell monolayers. Inflammation. 2014;38:375e383.
Chen S, Zhu J, Chen G, Zuo S, Zhang J, Chen Z, et al. 1,25-Dihydroxyvitamin D3 preserves intestinal epithelial barrier function from TNF-α induced injury via suppression of NF-kB p65 mediated MLCK-P-MLC signaling pathway. Biochem Biophys Res Commun. 2015;. doi:10.1016/j.bbrc.2015.03.125 (Epub ahead of print).
Zanardo RC, Brancaleone V, Distrutti E, et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–20.
Du J, Huang Y, Yan H, Zhang Q, Zhao M, et al. Hydrogen sulfide suppresses oxidized low-density lipoprotein (ox-LDL)-stimulated monocyte chemoattractant protein 1 generation from macrophages via the nuclear factor κB (NF-κB) pathway. J Biol Chem. 2014;289(14):9741–53.
Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137(2):569–78.
Flannigan KL, Agbor TA, Blackler RW, Kim JJ, Khan WI, Verdu EF, et al. Impaired hydrogen sulfide synthesis and IL-10 signaling underlie hyperhomocysteinemia-associated exacerbation of colitis. Proc Natl Acad Sci U S A. 2014;111(37):13559–64.
Medani M, Collins D, Docherty NG, Baird AW, O’Connell PR, Winter DC. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis. 2011;17(7):1620–5.
Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141(4):1323–33.
Puthia MK, Sio SW, Lu J, Tan KS. Blastocystis ratti induces contact- independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect Immun. 2006;74:4114.
Ma TY, Hoa NT, Tran DD, Bui V, Pedram A, Mills S, Merryfield M. Cyto- chalasin B modulation of Caco-2 tight junction barrier: role of myosin light chain kinase. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:875e885.
Ma TY, Nguyen D, Bui V, et al. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 1999;276:965e974.
Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enter- ocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013;182:375.
Schlegel N, Meir M, Spindler V, Germer CT, Waschke J. Differential role of RhoGTPases in intestinal epithelial barrier regulation in vitro. J Cell. Physi- ology. 2011;226:1196e1203.
Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol. 1982;92:79e91.
Siddiqui MR, Mayanil CS, Kim KS, Tomita T. Angiopoietin-1 Regulates Brain Endothelial Permeability through PTPN-2 Mediated Tyrosine Dephosphorylation of Occludin. PLoS ONE. 2015;10(6):e0130857.
Qasim M, Rahman H, Ahmed R, Oellerich M, Asif AR. Mycophenolic acid mediated disruption of the intestinal epithelial tight junctions Exp. Cell. Res. 2014;322:277e289.
Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell. Sci. 2006;119:2095e2106.
Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008;180:5653e5661.
Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation- induced diarrhea in vivo. J. Clin. Invest. 2005;115:2702e2715.
Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G422–30.
Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258:34–42.
Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G496–504.
Cao H, Zhou X, Zhang J, et al. Hydrogen sulfide protects against bleomycin-induced pulmonary fibrosis in rats by inhibiting NF-κB expression and regulating Th1/Th2 balance. Toxicol Lett. 2014;224(3):387–94.
Rao RK, Basuroy S, Rao VU, Karnaky KJ Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368(Pt 2):471–81.
Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, et al. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284(3):1559–69.
Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284(31):21036–46.
Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131(1):259–71.
Zanardo RC, Brancaleone V, Distrutti E, et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–20.
Wallace JL, Dicay M, McKnight W, et al. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–6.
Wallace JL, Blackler RW, Chan MV, et al. Anti-inflammatory and cytoprotective actions of hydrogen sulfide: translation to therapeutics. Antioxid Redox Signal. 2015;22:398–410.
Cao M, Wang P, Sun C, He W, Wang F. Amelioration of IFN-γ and TNF-α-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PLoS ONE. 2013;8(5):e61944.
Cheadle GA, Costantini TW, Lopez N, Bansal V, Eliceiri BP, Coimbra R. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS ONE. 2013;8(7):e69042.
Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumor necrosis factor-a induced regulation of myosin light chain kinase gene activity. J. Cell. Mol. Med. 2008;12:1331.
Boivin MA, Ye D, Kennedy JC, Al-Sadi R, Shepela C, Ma TY. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:590e598.
Moriez R, Salvador-Cartier C, Theodorou V, Fioramonti J, Eutamene H, Bueno L. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am. J. Pathol. 2005;167:1071e1079.
Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G422–30.
Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286(3):G367–76.
Guo C, Liang F, Shah Masood W, Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-κB dependent anti-inflammation pathway. Eur J Pharmacol. 2014;725:70–8.
Acknowledgments
We thank Professor Dingfang Pu for his excellent technical instructions in the measurement of Caco-2 monolayer permeability.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Funding
Funding for this work came from the China Health and Medical Development Foundation (Grant: Research on Surgical Infection Mechanism and Control).
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Chen, Sw., Zhu, J., Zuo, S. et al. Protective effect of hydrogen sulfide on TNF-α and IFN-γ-induced injury of intestinal epithelial barrier function in Caco-2 monolayers. Inflamm. Res. 64, 789–797 (2015). https://doi.org/10.1007/s00011-015-0862-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0862-5