Abstract
Objective and design
Sepsis refers to severe systemic inflammation in response to invading pathogens. To understand the molecular events that initiate the systemic inflammatory response, various inflammatory mediators were analyzed in neonatal sepsis samples and compared with normal samples.
Materials and methods
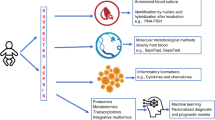
We initially measured the levels of the various classical inflammatory mediators such as acute phase proteins [C-reactive protein (CRP) and procalcitonin (PCT)], granule-associated mediators (NE, MPO and NO), proinflammatory cytokines [tumour necrosis factor-α (TNFα), IL-1β and IL-6), antiinflammatory cytokines (IL-10 and IL-13) and chemokines [IL-8 and monocyte chemotactic protein (MCP-1)] and novel cytokines (IL-12/IL-23p40, IL-21 and IL-23) using ELISA. We also used the human inflammation antibody array membrane to profile the inflammatory proteins that are involved in neonatal sepsis.
Results
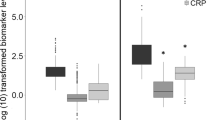
There were significantly higher levels of CRP (5.4 ± 0.70 mg/L), PCT (1.500 ± 0.2400 μg/L); NE (499.2 ± 22.01 μg/L), NO (54.22 ± 3.131 μM/L); TNFα (396.6 ± 37.40 pg/mL), IL-1β (445.3 ± 34.25 pg/mL), IL-6 (320.9 ± 43.38 pg/mL); IL-8 (429.5 ± 64.08 pg/mL) MCP-1 (626.25 ± 88.91 pg/mL), IL-10 (81.80 ± 9.45 pg/mL), IL-12/IL-23p40 (30.25 ± 0.6 pg/mL), IL-21 (8,263.3 ± 526.8 pg/mL) and IL-23 (6,083 ± 781.3 pg/mL) in neonates with sepsis compared to normal. The levels of MPO (21.20 ± 3.099 ng/mL) were downregulated, whereas there was no change in IL-13 (188.7 ± 10.63 pg/mL) levels in septic neonates when compared with normal. Using the human inflammation antibody array membrane, we detected the presence of 17 inflammatory proteins such as IL-3, IL6R, IL12p40, IL-16, TNFα, TNFβ, TNF R1, chemokines I-309, IP-10 (IFN-γ inducible protein 10), MCP-1, MCP-2, MIP 1β (macrophage inflammatory protein), MIP-1δ, eotaxin-2, growth factors TGFβ1 (transforming growth factor beta), PDGF (platelet derived growth factor), and cell adhesion molecule ICAM-1 (intracellular adhesion molecule) that were upregulated whereas RANTES which was downregulated in neonatal sepsis.
Conclusion
The simultaneous secretion and release of multiple mediators such as proinflammatory cytokines and chemokines, cell adhesion molecules, and growth factors were found to be involved in the initiation of systemic inflammation in neonatal sepsis. Therefore, measuring the concentration of multiple mediators may help in the early detection of neonatal sepsis and help to avoid unnecessary antibiotic treatment.




Similar content being viewed by others
References
Kenzel S, Henneke L. The innate immune system and its relevance to neonatal sepsis. Curr Opin Infect Dis. 2006;19(3):264–70.
Cloberty JP, Stark R, Eichenwald E. Manual of neonatal care. Chap 49, 7th ed. Lippincott Williams and Wilkins; 1998.
Pertova A, Metha R. Dysfunction of innate immunity and associated pathology in neonates. Indian J Pediatr. 2007;74:185–91.
Dasari P, Zola H, Nicholson IC. Expression of toll-like receptors by neonatal leukocytes. Pediatr Allergy Immunol. 2011;22(2):221–8.
Medzhitov R, Janeway CA Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8.
Hotoura E, Giapros V, Kostoula A, Spirou P, Andronikou S. Tracking changes of lymphocyte subsets and pre-inflammatory mediators in full-term neonates with suspected or documented infection. Scand J Immunol. 2011;73(3):250–5.
Santana RC, Garcia MF, Reyes D, Gonzalez G, Dominguez C, Domenech E. Role of cytokines (interleukin-1beta, 6, 8, tumour necrosis factor-alpha, and soluble receptor of interleukin-2) and C-reactive protein in the diagnosis of neonatal sepsis. Acta Paediatr. 2003;92(2):221–7.
Abdelhamid AE, Chuang SL, Hayes P, Fell JM. In vitro cow’s milk protein-specific inflammatory and regulatory cytokine responses in preterm infants with necrotizing enterocolitis and sepsis. Pediatr Res. 2011;69(2):165–9.
Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaeghel G, Moutardier V, Blache JL. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005;94(6):767–73.
Weinberg GA, D’Angio CT. The search for new diagnostic tests for neonatal sepsis. J Pediatr. 2009;155(5):763–4.
Riedemann NC, Goo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–20.
Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013. doi:10.1189/jlb.0912437.
Miller E. Phagocytosis in the newborn infant. J Ped. 1969;74:255.
Baltimore R, Huie SM, Meek JI, Schuchat A, Brien KLO. Early-onset neonatal sepsis in the era of group B Streptococcal prevention. J Pediatr. 2001;108:1094–8.
Baltimore RS. Perinatal bacterial and fungal infections. In: Jenson HB, Baltimore RS, editors. Ped Inf Dis. Prin Prac. 2nd ed. Philadelphia: WB Saunders Co; 2002. p. 1119–34.
Dollner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J Clin Epidemiol. 2001;54:1251.
Chiesa C, Natale F, Pascone R, Osborn JF, Pacifico L, Bonci E, De Curtis M. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chimica Acta. 2011;412:1053–9.
Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophils cells. J Ped. 1979;95(1):89–98.
Tsaka T, Herkner KR. Polymorphonuclear elastase in neonatal sepsis. Clin Chim Acta. 1990;193:103–12.
Henson PM. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971;107:1535.
Ohlsson K, Olsson AS. Immunoreactive granulocyte elastase in human serum. Hoppe-Seyler’s Z Physiol Chem. 1978;359:1531.
Terregino C, Lopez B, Karras D, Killian A, Arnold G. Endogenous mediators in emergency department patients with presumed sepsis: are levels associated with progression to severe sepsis and death. Ann Emerg Med. 2000;35:26–34.
Shi YH, Li C, Shen J, Wang S, Qin R, Pan LJ. Plasma nitric oxide level in newborn infants with sepsis. J Ped. 1993;123:436–8.
Spack LP, Griffith HO. Measurement of total plasma nitrite and nitrate in pediatric patients with the systemic inflammatory response syndrome. Crit Care Med. 1997;25:1071–8.
Martin H, Olander B, Norman M. Reactive hyperemia and interleukin 6, interleukin 8, and tumor necrosis factor-α in the diagnosis of early-onset neonatal sepsis. Pediatrics. 2001;108:1–6.
Urbonas V, Eidukaite A, Tamuliene I. Increased interleukin-10 levels correlate with bacteremia and sepsis in febrile neutropenia pediatric oncology patients. Cytokine. 2012;57:313–5.
Takahashi N, Uehara R, Kobayashi M, Yada Y, Koike Y, Kawamata R, Odaka J, Honma Y, Momoi MY. Cytokine profiles of seventeen cytokines, growth factors and chemokines in cord blood and its relation to perinatal clinical findings. Cytokine. 2010;49:331–7.
Caoa YZ, Tua YY, Chen X, Wang BL, Zhong YX, Liu MH. Protective effect of ulinastatin against murine models of sepsis: inhibition of TNF and IL-6 and augmentation of IL-10 and IL-13. Exp Toxicol Pathol. 2012;64(6):543–7.
Schelonka RL, Maheshwari A, Carlo WA, Taylor S, Hansen NI, Schendel DE, Thorsen P, Skogstrand K, Hougaard DM, Higgins RD, NICHD Neonatal Research Network. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine. 2011;53:249–55.
Ng PC, Li K, Leung TF, Wong RP, Li G, Chui KM, Wong E, Cheng FW, Fok TF. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52(6):1181–9.
Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcovici A, Zanc V, et al. Multiplex cytokine profiling in patients with sepsis. Apmis. 2011;119(2):155–63.
Andaluz-Ojeda D, Bobillo F, Iglesias V, Almansa R, Rico L, Gandía F, Resino S, Tamayo E, de Lejarazu RO, Bermejo-Martin JF. A combined score of pro- and anti inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57(3):332–6.
Harlan JM. Leukocyte–endothelial interactions. Blood. 1985;65:513–25.
Manoura A, Gourgiotis D, Galanakis E, Matalliotakis E, Hatzidaki E, Korakaki E, Saitakis E, Marmarinos AS, Giannakopoulou C. Circulating concentrations of α- and β-chemokines in neonatal sepsis. Int J Infect Dis. 2010;14(9):e806–9.
Wu YH, Chuang SY, Hong WC, Lai YJ, Chang YL, Pang JH. In vivo and in vitro inhibitory effects of a traditional Chinese formulation on LPS-stimulated leukocyte–endothelial cell adhesion and VCAM-1 gene expression. J Ethnopharmacol. 2012;140(1):55–63.
Cheon H, Yu SJ, Yoo DH, Chae IJ, Song GG, Sohn J. Increased expression of pro-inflammatory cytokines and metalloproteinase-1 by TGFbeta1 in synovial fibroblasts from rheumatoid arthritis and normal individuals. Clin Exp Immunol. 2002;127:547–52.
Edwards DR, Leco KJ, Beaudry PP, Atadja PW, Veillette C, Riabowol KT. Differential effects of transforming growth factor-beta 1 on the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in young and old human fibroblasts. Exp Gerontol. 1996;31:207–23.
Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, et al. Transforming growth factor beta 1(TGFbeta 1) induced neutrophil recruitment to synovial tissues: implications for TGFbeta-driven synovial inflammation and hyperplasia. J Exp Med. 1991;173(5):1121–32.
Ramsay PL, O’Brian Smith E, Hegemier S, et al. Early clinical markers for the development of bronchopulmonary dysplasia: soluble E-Selectin and ICAM-1. Pediatrics. 1998;102:927.
Endo S, Inada K, Kasai T, et al. Levels of soluble adhesion molecules and cytokines in patients with septic multiple organ failure. J Inflamm. 1995;46:212.
Xanthou M, Fotopoulos S, Mouchtouri A, et al. Inflammatory mediators in perinatal asphyxia and infection. Acta Paediatr Suppl. 2002;91:92.
Ramnath RD, Ng SW, Guglielmotti A, Bhatia M. Role of MCP-1 in endotoxemia and sepsis. Int Immunopharmacol. 2008;8:810–8.
Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49.
Kantar M, Kültürsay N, Kütükçüler N, Akisü M, Cetingül N, Caglayan S. Plasma concentrations of granulocyte-macrophage colony-stimulating factor and interleukin-6 in septic and healthy preterms. Eur J Pediatr. 2000;159:156–7.
Magudumana O, Ballot DE, Cooper PA, et al. Serial interleukin 6 measurements in the early diagnosis of neonatal sepsis. J Trop Pediatr. 2000;46:267–71.
Yannam GR, Gutti T, Poluektova LY. IL-23 in infections, inflammation, autoimmunity and cancer: possible role in HIV-1 and AIDS. J Neuroimmune Pharmacol. 2012;7:95–112.
Louis S, Dutertre C-A, Vimeux L, Fery L, Henno L, Diocou S, Kahi S, Deveau C, Meyer L, Goujard C, Hosmalin A. IL-23 and IL-12p70 production by monocytes and dendritic cells in primary HIV-1 infection. J Leukoc Biol. 2010;. doi:10.1189/jlb.1009684.
Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183(11):7150–60.
Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49.
Nakano N, Nishiyama C, Kanada S, Niwa Y, Shimokawa N, Ushio H, Nishiyama M, Okumura K, Ogawa H. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood. 2007;109:4846–55.
Acknowledgments
This study is supported by the grant from Department of Biotechnology (DBT), Government of India (No. BT/PR12666/BRB/10/723/2009). The authors are thankful to SRM University for their support. Authors are also thankful to staffs of SRM hospital and CMC hospital, Chengalpet for assistance in sample collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Sugitharini, V., Prema, A. & Berla Thangam, E. Inflammatory mediators of systemic inflammation in neonatal sepsis. Inflamm. Res. 62, 1025–1034 (2013). https://doi.org/10.1007/s00011-013-0661-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0661-9