Abstract
Objective
The roles that amino acids play in immunity and inflammation are well defined, and the relationship between inflammatory bowel disease (IBD) and certain amino acids has recently attracted attention. In this study, the levels of amino acids and trichloroacetic acid (TCA) cycle-related molecules in the colonic tissues and sera of patients with ulcerative colitis (UC) were profiled by gas chromatography/mass spectrometry (GC/MS), with the aim of evaluating whether the clinical state induced by UC leads to variations in the amino acid profile.
Materials and methods
Colonic biopsy samples from 22 UC patients were used, as well as serum samples from UC patients (n = 13), Crohn’s disease (CD) patients (n = 21), and healthy volunteers (n = 17).
Results
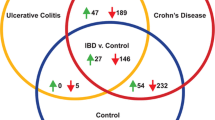
In the GC/MS-based profiling of amino acids and TCA cycle-related molecules, lower levels of 16 amino acids and 5 TCA cycle-related molecules were observed in the colonic lesion tissues of the UC patients, and the serum profiles of amino acids and TCA cycle-related molecules of the UC patients were different from those of the CD patients and healthy volunteers.
Conclusions
Our study raises the possibility that GC/MS-based profiling of amino acids and TCA cycle-related molecules is a useful early diagnostic tool for UC.


Similar content being viewed by others
Abbreviations
- IBD:
-
Inflammatory bowel disease
- CD:
-
Crohn’s disease
- UC:
-
Ulcerative colitis
- ED:
-
Elementary diet
- NMR:
-
Nuclear magnetic resonance analysis
- GC/MS:
-
Gas chromatography/mass spectrometry
- LC/MS:
-
Liquid chromatography/mass spectrometry
- CE/MS:
-
Capillary electrophoresis/mass spectrometry
- TCA:
-
Trichloroacetic acid
- CAI:
-
Clinical activity index
- IOIBD:
-
International Organization for the Study of Inflammatory Bowel Disease
- MSTFA:
-
N-Methyl-N-trimethylsilyl-trifluoroacetamide
- PCA:
-
Principal component analysis
- PLS-DA:
-
Partial least squares discriminant analysis
References
Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology. 2003;124:521–36.
Katz JA, Itoh J, Fiocchi C. Pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 1999;15:291–7.
Young Y, Abreu MT. Advances in the pathogenesis of inflammatory bowel disease. Curr Gastroenterol. 2006;8:470–7.
Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schütz T, van Gemert W, van Gossum A, Valentini L, Lübke H, Bischoff S, Engelmann N, Thul P. ESPEN guidelines on enteral nutrition: gastroenterology. Clin Nutr. 2006;25:260–74.
Homan M, Baldassano RN, Mamula P. Managing complicated Crohn’s disease in children and adolescents. Nat Clin Pract Gastroenterol Hepatol. 2005;2:572–9.
Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut. 2006;55:356–61.
Yamamoto T, Nakahigashi M, Saniabadi AR. Review article: diet and inflammatory bowel disease-epidemiology and treatment. Aliment Pharmacol Ther. 2009;30:99–112.
Matsui T, Sakurai T, Yao T. Nutritional therapy for Crohn’s disease in Japan. J Gastroenterol. 2005;40:25–31.
Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A, Yamamoto S. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumor necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–93.
Israeli E, Berenshtein E, Wengrower D, Aptekar L, Kohen R, Zajicek G, Goldin E. Prophylactic administration of topical glutamine enhances the capability of the rat colon to resist inflammatory damage. Dig Dis Sci. 2004;49:1705–12.
Giriş M, Erbil Y, Doğru-Abbasoğlu S, Yanik BT, Aliş H, Olgaç V, Toker GA. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. The effect of glutamine on TNBS colitis. Int J Colorectal Dis. 2007;22:591–9.
Vicario M, Amat C, Rivero M, Moretó M, Pelegrí C. Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J Nutr. 2007;137:1931–7.
Arndt H, Kullmann F, Reuss F, Schölmerich J, Palitzsch KD. Glutamine attenuates leukocyte endothelial cell adhesion in indomethacin-induced intestinal inflammation in the rat. JPEN J Parenter Enteral Nutr. 1999;23:12–8.
Basivireddy J, Jakob M, Balasubramanian KA. Oral glutamine attenuates indomethacin-induced small intestinal damage. Clin Sci. 2004;107:281–9.
Fillmann H, Kretzmann NA, San-Miguel B, Llesuy S, Marroni N, González-Gallego J, Tuñón MJ. Glutamine inhibits over-expression of pro-inflammatory genes and down-regulates the nuclear factor kappaB pathway in an experimental model of colitis in the rat. Toxicology. 2007;236:217–26.
Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, Sato N. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125:775–85.
Andou A, Hisamatsu T, Okamoto S, Chinen H, Kamada N, Kobayashi T, Hashimoto M, Okutsu T, Shimbo K, Takeda T, Matsumoto H, Sato A, Ohtsu H, Suzuki M, Hibi T. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology. 2009;136:564–74.
Bjerrum JT, Nielsen OH, Wang YL, Olsen J. Technology insight: metabonomics in gastroenterology-basic principles and potential clinical applications. Nat Clin Pract Gastroenterol Hepatol. 2008;5:332–43.
Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, Soga T. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–25.
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–4.
Gao H, Lu Q, Liu X, Cong H, Zhao L, Wang H, Lin D. Application of 1H NMR-based metabonomics in the study of metabolic profiling of human hepatocellular carcinoma and liver cirrhosis. Cancer Sci. 2009;100:782–5.
Marion JF, Rubin PH, Present DH. Differential diagnosis of chronic ulcerative colitis and Crohn’s disease. In: Kirsner JB, editor. Inflammatory bowel disease. 5th ed. Philadelphia: WB Saunders; 2000. p. 315–25.
Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–6.
Myren J, Bouchier IA, Watkinson G, Softley A, Clamp SE, de Dombal FT. The O.M.G.E. multinational inflammatory bowel disease survey 1976–1982. A further report on 2,657 cases. Scand J Gastroenterol. 1984;19:1–27.
Nishiumi S, Shinohara M, Ikeda A, Yoshie T, Hatano N, Kakuyama S, Mizuno S, Sanuki T, Kutsumi H, Fukusaki E, Azuma T, Takenawa T, Yoshida M. Serum metabolomics as a novel diagnostic approach for pancreatic cancer. Metabolomics. 2010;6:518–28.
Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med. 1961;30:393–407.
Shiomi Y, Nishiumi S, Ooi M, Hatano N, Shinohara M, Yoshie T, Kondo Y, Furumatsu K, Shiomi H, Kutsumi H, Azuma T, Yoshida M. A GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm Bowel Dis 2011 (in press) doi:10.1002/ibd.21616.
Fernández-Bañares F, Cabré E, González-Huix F, Gassull MA. Enteral nutrition as primary therapy in Crohn’s disease. Gut. 1994;35:55–9.
Lennie TA, McCarthy DO, Keesey RE. Body energy status and the metabolic response to acute inflammation. Am J Physiol. 1995;269:1024–31.
Ling PR, Bistrian BR, Mendez B, Istfan NW. Effects of systemic infusions of endotoxin, tumor necrosis factor, and, interleukin-1 on glucose metabolism in the rat: relationship to endogenous glucose production and peripheral tissue glucose uptake. Metabolism. 1994;43:279–84.
Jijon HB, Churchill T, Malfair D, Wessler A, Jewell LD, Parsons HG, Madsen KL. Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am J Physiol Gastointest Liver Physiol. 2000;279:641–51.
Cetinkaya A, Bulbuloglu E, Kantarceken B, Ciralik H, Kurutas EB, Buyukbese MA, Gumusalan Y. Effects of l-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci. 2006;51:488–94.
Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1964;5:1–22.
Goh J, O’Morain CA. Nutrition and adult inflammatory bowel disease. Aliment Pharmacol Ther. 2003;17:307–20.
Rizzello F, Gionchetti P, Venturi A, Campieri M. Review article: medical treatment of severe ulcerative colitis. Aliment Pharmacol Ther. 2003;17:7–10.
Coëffier M, Déchelotte P. The role of glutamine in intensive care unit patients: mechanisms of action and clinical outcome. Nutr Rev. 2005;63:65–9.
Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17.
Wu F, Dassopoulos T, Cope L, Maitra A, Brant SR, Harris ML, Bayless TM, Parmigiani G, Chakravarti S. Genome-wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflamm Bowel Dis. 2007;13:807–21.
Olsen J, Gerds TA, Seidelin JB, Csillag C, Bjerrum JT, Troelsen JT, Nielsen OH. Diagnosis of ulcerative colitis before onset of inflammation by multivariate modeling of genome-wide gene expression data. Inflamm Bowel Dis. 2009;15:1032–8.
Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–12.
Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661–5.
Lennard-Jones JE, Lockhart-Mummery HE, Morson BC. Clinical and pathological differentiation of Crohn’s disease and proctocolitis. Gastroenterology. 1968;54:1162–70.
Hoffenberg EJ, Fidanza S, Sauaia A. Serologic testing for inflammatory bowel disease. J Pediatr. 1999;134:447–52.
Peeters M, Joossens S, Vermeire S, Vlietinck R, Bossuyt X, Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–4.
Dubinsky MC, Ofman JJ, Urman M, Targan SR, Seidman EG. Clinical utility of serodiagnostic testing in suspected pediatric inflammatory bowel disease. Am J Gastroenterol. 2001;96:758–65.
Sandborn WJ, Loftus EV Jr, Colombel JF, Fleming KA, Seibold F, Homburger HA, Sendid B, Chapman RW, Tremaine WJ, Kaul DK, Wallace J, Harmsen WS, Zinsmeister AR, Targan SR. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2001;7:192–201.
Acknowledgments
Grant support: T.Y., T.A., and M.Y. were supported by a grant from the Global COE Program “Global Center of Excellence for Education and Research on Signal Transduction Medicine in the Coming Generation” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. S.N. and T.A. were partially supported by a grant from the “Young researchers training program for promoting innovation” program of the Special Coordination Fund for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Y.S. was partially supported by a grant from the Education Program for Specialized Clinicians in the Support Program for Improving Graduate School Education from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank Kazuko Nagase (The Divisions of Lower Gastroenterology, Department of Internal Medicine, Hyogo College of Medicine) for sample collection and preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ian Ahnfelt-Rønne.
M. Ooi and S. Nishiumi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ooi, M., Nishiumi, S., Yoshie, T. et al. GC/MS-based profiling of amino acids and TCA cycle-related molecules in ulcerative colitis. Inflamm. Res. 60, 831–840 (2011). https://doi.org/10.1007/s00011-011-0340-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0340-7