Abstract
Objective and design
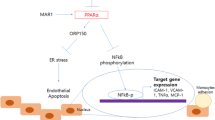
The pro-oxidative and pro-inflammatory pathways in vascular endothelium have been implicated in the development of atherosclerosis. In the present study, we investigated effect of interleukin-4 (IL-4) on monocyte chemoattractant protein-1 (MCP-1) expression in vascular endothelium and examined the role of distinct sources of reactive oxygen species (ROS) in this process.
Methods and results
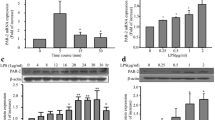
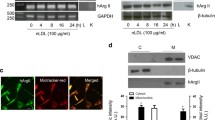
Real-time reverse transcriptase-polymerase chain reaction and enzyme-linked immunosorbent assay showed that IL-4 significantly up-regulated mRNA and protein expression of MCP-1 in human aortic endothelial cells (HAEC) and C57BL/6 mice. A significant and dose-dependent inhibition of IL-4-induced MCP-1 expression was observed in HAEC pre-treated with antioxidants, such as pyrrolidine dithiocarbamate and epigallocatechin gallate, indicating that IL-4-induced MCP-1 expression is mediated via a ROS-dependent mechanism. Additionally, pharmacological inhibitors of NADPH oxidase (NOX) significantly attenuated IL-4-induced MCP-1 expression in HAEC. Furthermore, the disruption of the NOX gene dramatically reduced IL-4-induced MCP-1 expression in NOX knockout mice (B6.129S6-Cybbtm1Din/J). In contrast, overexpression of MCP-1 in IL-4-stimulated HAEC was not affected by inhibiting other ROS generating pathways, such as xanthine oxidase and the mitochondrial electron transport chain.
Conclusions
These results demonstrate that IL-4 up-regulates MCP-1 expression in vascular endothelium through NOX-mediated ROS generation.










Similar content being viewed by others
References
Lee YW, Hennig B, Toborek M. Redox-regulated mechanisms of interleukin-4-induced MCP-1 expression in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H185–92.
Rollins BJ, Pober JS. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991;138:1315–9.
Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–8.
Strieter RM, Wiggins R, Phan SH, Wharram BL, Showell HJ, Remick DG, et al. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989;162:694–700.
Taubman MB, Rollins BJ, Poon M, Marmur J, Green RS, Berk BC, et al. JE mRNA accumulates rapidly in aortic injury and in platelet-derived growth factor-stimulated vascular smooth muscle cells. Circ Res. 1992;70:314–25.
Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81:1–7.
Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–7.
Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard EJ, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA. 1991;88:5252–6.
Takeya M, Yoshimura T, Leonard EJ, Takahashi K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993;24:534–9.
Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–81.
Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–8.
Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–7.
Aiello RJ, Bourassa PK, Lindsey S, Weng W, Natoli E, Rollins BJ, et al. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–25.
Martinovic I, Abegunewardene N, Seul M, Vosseler M, Horstick G, Buerke M, et al. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circ J. 2005;69:1484–9.
Ni W, Egashira K, Kitamoto S, Kataoka C, Koyanagi M, Inoue S, et al. New anti-monocyte chemoattractant protein-1 gene therapy attenuates atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2001;103:2096–101.
Inoue S, Egashira K, Ni W, Kitamoto S, Usui M, Otani K, et al. Anti-monocyte chemoattractant protein-1 gene therapy limits progression and destabilization of established atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2002;106:2700–6.
Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81.
Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-γ on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–60.
Barks JL, McQuillan JJ, Iademarco F. TNF-α and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol. 1997;159:4532–87.
Blease K, Seybold J, Adcock IM, Hellewell PG, Burke-Gaffney A. Interleukin-4 and lipopolysaccharide synergize to induce vascular cell adhesion molecule-1 expression in human lung microvascular endothelial cells. Am J Respir Cell Mol Biol. 1998;18:620–30.
Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–25.
Galéa P, Thibault G, Lacord M, Bardos P, Lebranchu Y. IL-4, but not tumor necrosis factor-alpha, increases endothelial cell adhesiveness for lymphocytes by activating a cAMP-dependent pathway. J Immunol. 1993;151:588–96.
Hong HY, Lee HY, Kwak W, Yoo J, Na MH, So IS, et al. Phage display selection of peptides that home to atherosclerotic plaques: IL-4 receptor as a candidate target in atherosclerosis. J Cell Moll Med. 2008;12:2003–14.
Huang H, Lavoie-Lamoureux A, Moran K, Lavoie JP. IL-4 stimulates the expression of CXCL-8, E-selectin, VEGF, and inducible nitric oxide synthase mRNA by equine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1147–54.
King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22:456–61.
Lee YW, Eum SY, Chen KC, Hennig B, Toborek M. Gene expression profile in interleukin-4-stimulated human vascular endothelial cells. Mol Med. 2004;10:19–27.
Lee YW, Hirani AA. Role of interleukin-4 in atherosclerosis. Arch Pharm Res. 2006;29:1–15.
Lee YW, Kühn H, Hennig B, Neish AS, Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J Mol Cell Cardiol. 2001;33:83–94.
Lee YW, Kühn H, Kaiser S, Hennig B, Daughterty A, Toborek M. Interleukin 4 induces transcription of the 15-lipoxygenase I gene in human endothelial cells. J Lipid Res. 2001;42:783–91.
Masinovsky B, Urdal D, Gallatin WM. IL-4 acts synergistically with IL-1β to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990;145:2886–95.
Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70.
Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2 − and systolic blood pressure in mice. Circ Res. 2001;89:408–11.
Rocken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:225–31.
Walch L, Massade L, Dufilho M, Brunet A, Rendu F. Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis. 2006;187:285–91.
Lee YW, Lee WH, Kim PH. Oxidative mechanisms of IL-4-induced IL-6 expression in vascular endothelium. Cytokine. 2010;49:73–9.
Lee YW, Kühn H, Hennig B, Daughterty A, Toborek M. IL-4 induces apoptosis of endothelial cells through the caspase-3-dependent pathway. FEBS Lett. 2000;485:122–6.
Sasaguri T, Arima N, Tanimoto A, Shimajiri S, Hamada T, Sasaguri Y. A role for interleukin 4 in production of matrix metalloproteinase 1 by human aortic smooth muscle cells. Atherosclerosis. 1998;138:247–53.
Chen CC, Manning AM. TGF-β1, IL-10 and IL-4 differentially modulate the cytokine-induced expression of IL-6 and IL-8 in human endothelial cells. Cytokine. 1996;8:58–65.
Bennett BL, Cruz R, Lacson RG, Manning AM. Interleukin-4 suppression of tumor necrosis factor α-stimulated E-selectin gene transcription is mediated by STAT6 antagonism of NF-κB. J Biol Chem. 1997;272:10212–9.
Wright PS, Cooper JR, Kropp KE, Busch SJ. Induction of vascular cell adhesion molecule-1 expression by IL-4 in human aortic endothelial cells is not associated with increased nuclear NF-κB levels. J Cell Physiol. 1999;180:381–9.
Toborek M, Lee YW, Kaiser S, Hennig B. Measurement of inflammatory properties of fatty acids in human endothelial cells. Methods Enzymol. 2002;352:198–219.
Deng X, Li H, Tang YW. Cytokine expression in respiratory syncytial virus-infected mice as measured by quantitative reverse-transcriptase PCR. J Virol Methods. 2003;107:141–6.
Lee YW, Lee WH. Protective effects of genistein on pro-inflammatory pathways in human brain microvascular endothelial cells. J Nutr Biochem. 2008;19:819–25.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods. 2001;25:402–8.
Rollins BJ. Chemokines. Blood. 1997;90:909–28.
Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol. 1999;72:7–29.
Bursill CA, Channon KM, Greaves DR. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr Opin Lipidol. 2004;15:145–9.
Sheikine YA, Hansson GK. Chemokines as potential therapeutic targets in atherosclerosis. Curr Drug Targets. 2006;7:13–27.
Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–21.
Gimbrone MA, Bevilacqua MP, Cybulsky MI. Endothelial-dependent mechanisms of leukocyte adhesion in inflammation and atherosclerosis. Ann NY Acad Sci. 1990;598:77–85.
Iseki A, Kambe F, Okumura K, Niwata S, Yamamoto R, Hayakawa T, et al. Pyrrolidine dithiocarbamate inhibits TNF-α-dependent activation of NF-κB by increasing intracellular copper level in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2000;276:88–92.
Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7:135–44.
Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25:1401–7.
Hennig B, Toborek M, McClain CJ, Diana JN. Nutritional implications in vascular endothelial cell metabolism. J Am Coll Nutr. 1996;15:345–58.
Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–35.
Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, et al. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–9.
Hennig B, Chow CK. Lipid peroxidation and endothelial cells injury: implication in atherosclerosis. Free Radical Biol Med. 1988;4:99–106.
Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–65.
Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8.
Yla-Herttuala S. Gene expression in atherosclerotic lesions. Hertz. 1992;17:270–6.
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9.
Brinckmann R, Topp MS, Zalan I, Heydeck D, Ludwig P, Kühn H, et al. Regulation of 15-lipoxygenase expression in lung epithelial cells by interleukin-4. Biochem J. 1996;318:305–12.
Bedard K, Krause K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Acknowledgments
This study was supported in part by Grants from National Institutes of Health/National Heart, Lung, and Blood Institute (HL085229) and National Science Foundation Macromolecular Interfaces with Life Sciences-Integrative Graduate Education and Research Traineeship (MILES-IGERT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: I. Ahnfelt-Rønne.
Rights and permissions
About this article
Cite this article
Lee, Y.W., Lee, W.H. & Kim, P.H. Role of NADPH oxidase in interleukin-4-induced monocyte chemoattractant protein-1 expression in vascular endothelium. Inflamm. Res. 59, 755–765 (2010). https://doi.org/10.1007/s00011-010-0187-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0187-3