Abstract
Objective and design
To investigate the role of non-protein sulfhydryl groups (NP-SH) and leukocyte adhesion in the protective effect of lipopolysaccharide (LPS) from Escherichia coli against indomethacin-induced gastropathy.
Materials or subjects
Male Wistar rats were divided into four groups: saline, LPS, saline + indomethacin and LPS + indomethacin, with six rats in each group.
Treatment
Rats were pretreated with LPS (300 μg/kg, by intravenous) or saline. After 6 h, indomethacin was administered (20 mg/kg, by gavage).
Methods
Three hours after treatments, rats were killed. Macroscopic gastric damage, gastric NP-SH concentration, myeloperoxidase (MPO) activity and mesenteric leukocyte adhesion (intravital microscopy) were assessed. Statistical analysis was performed using one-way analysis of variance followed by the Newman–Keuls test. Statistical significance was set at P < 0.05.
Results
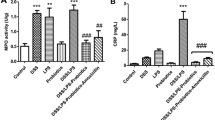
LPS reduced the gastric damage, gastric MPO activity and increased gastric NP-SH concentration in indomethacin-induced gastropathy. LPS alone increased gastric NP-SH when compared to saline. Indomethacin increased leukocyte adhesion when compared to the saline, and LPS reduced indomethacin-induced leukocyte adhesion. In addition, LPS alone did not change leukocyte adhesion, when compared to the saline.
Conclusion
LPS protective effect against indomethacin-induced gastropathy is mediated by an increase in the NP-SH and a decrease in leukocyte–endothelial adhesion.




Similar content being viewed by others
Abbreviations
- LPS:
-
Lipopolysaccharide
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- INDO:
-
Indomethacin
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- iNOS:
-
Inducible nitric oxide synthase
- MPO:
-
Myeloperoxidase
- NP-SH:
-
Non-protein sulfhydryl groups
References
Wallace JL. Mechanisms of non-steroidal anti-inflammatory drug (NSAID) induced gastrointestinal damage potential for development of gastrointestinal tract safe NSAIDs. Can J Physiol Pharmacol. 1994;72(12):1493–8.
Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by non-steroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990;259:462–7.
Wallace JL. Gastric ulceration: critical events at the neutrophil endothelium interface. Can J Physiol Pharmacol. 1993;71(1):98–102.
Castaneda AA, Denning JW, Chang L, Mercer DW. Does up-regulation of inducible nitric oxide synthase (iNOS) render the stomach more susceptible to damage? J Surg Res. 1999;84:174–9.
Ferraz JG, Sharkey KA, Reuter BK, Asfaha S, Tigley AW, Brown ML, et al. Induction of cyclooxygenase 1 and 2 in the rat stomach during endotoxemia: role in resistance to damage. Gastroenterology. 1997;113:195–204.
Konturek PC, Brzozowski T, Meixner H, Ptak A, Hahn EG, Konturek SJ. Central and peripheral neural aspects of gastroprotective and ulcer healing effects of lipopolysaccharides. J Physiol Pharmacol. 2001;52:611–23.
Gomes AS, Lima LM, Santos CL, Cunha FQ, Ribeiro RA, Souza MH. LPS from Escherichia coli protects against indomethacin-induced gastropathy in rats—role of ATP-sensitive potassium channels. Eur J Pharmacol. 2006;547(1–3):136–42.
Vaananen PM, Meddings JB, Wallace JL. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am J Physiol. 1991;261:470–5.
Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27(9–10):916–21.
Zhu Y, Carvey PM, Ling Z. Altered glutathione homeostasis in animals prenatally exposed to lipopolysaccharide. Neurochem Int. 2007;50(4):671–80. Epub Jan 13.
Haddad JJ, Harb HL. l-Gamma-glutamyl-l-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol Immunol. 2005;42(9):987–1014. Epub Nov 23.
Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A. Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: role of tumour necrosis factor alpha. Gut. 1994;35(7):909–15.
Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–22.
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25(1):192–205.
Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–8.
Baez S. Simultaneous measurements of radii and wall thickness of microvessels in the anesthetized rat. Circ Res. 1969;25(3):315–29.
Szabo S, Trier JS, Frankel PW. Sulfhydryl compounds may mediate gastric cytoprotection. Science. 1981;214(4517):200–2.
Souza MH, Troncon LE, Cunha FQ, Oliveira RB. Decreased gastric tone and delayed gastric emptying precede neutrophil infiltration and mucosal lesion formation in indomethacin-induced gastric damage in rats. Braz J Med Biol Res. 2003;36:1383–90.
Ueshima K, Takeuchi K, Okabe S. Effects of sulfhydryl-related compounds on indomethacin-induced gastric lesions in rats: role of endogenous sulfhydryls in the pathogenesis. Jpn J Pharmacol. 1992;58(2):157–65.
Mota JM, Soares PM, Menezes AA, Lemos HP, Cunha FQ, Brito GA, et al. Amifostine (Wr-2721) prevents indomethacin-induced gastric damage in rats: role of non-protein sulfhydryl groups and leukocyte adherence. Dig Dis Sci. 2007;52(1):119–25.
Kenchappa RS, Ravindranath V. Gamma-glutamyl cysteine synthetase is up-regulated during recovery of brain mitochondrial complex I following neurotoxic insult in mice. Neurosci Lett. 2003;350(1):51–5.
Morise Z, Granger DN, Fuseler JW, Anderson DC, Grisham MB. Indomethacin induced gastropathy in CD18, intercellular adhesion molecule 1, or P-selectin deficient mice. Gut. 1999;45(4):523–8.
Rosenbaum AJ, Murphy PJ, Engel JJ. Pleurisy during the course of ulcerative colitis. J Clin Gastroenterol. 1983;5(6):517–21.
Cunha FQ, Souza GE, Souza CA, Cerqueira BC, Ferreira SH. In vivo blockage of neutrophil migration by LPS is mimicked by a factor released from LPS-stimulated macrophages. Br J Exp Pathol. 1989;70:1–8.
Thomazzi SM, Souza MH, Melo-Filho AA, Hewlett EL, Lima AA, Ribeiro RA. Pertussis toxin from Bordetella pertussis blocks neutrophil migration and neutrophil-dependent edema in response to inflammation. Braz J Med Biol Res. 1995;28:120–4.
Benjamim CF, Ferreira SH, Cunha FQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis. 2000;182:214–23.
Crosara-Alberto DP, Darini AL, Inoue RY, Silva JS, Ferreira SH, Cunha FQ. Involvement of NO in the failure of neutrophil migration in sepsis induced by Staphylococcus aureus. Br J Pharmacol. 2002;136(5):645–58.
Alves-Filho JC, Benjamim C, Tavares-Murta BM, Cunha FQ. Failure of neutrophil migration toward infectious focus in severe sepsis: a critical event for the outcome of this syndrome. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):223–6.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Maria Silvandira Freire França. Grants from CNPq (Brazil) supported this work. Dr. Cunha and Dr. Souza are recipients of a CNPq fellowship. This work is part of the requirements to obtain a Master of Science degree in Pharmacology at the School of Medicine, Federal University of Ceara by one of us (A.S. Gomes).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: I. Ahnfelt-Rønne.
Rights and permissions
About this article
Cite this article
Gomes, A.S., Lemos, H.P., Medeiros, J.V.R. et al. Lipopolysaccharide from Escherichia coli prevents indomethacin-induced gastric damage in rats: role of non-protein sulfhydryl groups and leukocyte adherence. Inflamm. Res. 58, 717–723 (2009). https://doi.org/10.1007/s00011-009-0040-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-009-0040-8