Abstract
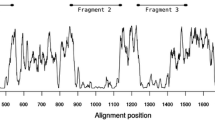
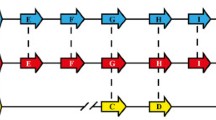
Eleven new alleles of the Plasmodium falciparum merozoite surface antigen 2 (MSA2) from Papua New Guinea were analyzed by direct sequencing of polymerase chain reaction (PCR) products. We have used the sequence information to trace the molecular evolution of MSA2. The repeats of ten alleles belonging to the 3D7 allelic family differed considerably in size, nucleotide sequence, and repeat copy number. In the repeat region of these new alleles, codon usage was extremely biased with an exclusive use of NNT codons. Another new allele sequenced belonged to the FC27 family and confirmed the family-specific conserved structure of 96 and 36 bp repeats. In order to assess sequence microheterogeneity within samples defined as the same genotype by restriction fragment length polymorphism (RFLP), we have analyzed single-strand conformation polymorphism (SSCP) of different samples of the most frequent allele (D10 of the FC27 family) in the study population. No sequence heterogeneity could be detected within the repeat region. Based on analysis of the repeat regions in both allelic families, we discuss the hypothesis of a different evolutionary strategy being represented by each of the allelic families.
Similar content being viewed by others
References
Al-Yaman F, Genton B, Anders RF, Falk M, Triglia T, Lewis D, Hii J, Beck H-P, Alpers M (1994) Relationship between humoral response to merozoite surface antigen 2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg 51:593–602
Alpers MP, AI-Yaman F, Beck H-P, Bhatia KK, Hii J, Lewis DJ, Paru R, Smith T (1992) The Malaria Vaccine Epidemiology and Evaluation Project of Papua New Guinea: rationale and baseline studies. PNG Med J 35:285–297
Arnot DE, Barnwell JW, Stewart MJ (1988) Does biased gene conversion influence polymorphism in the circumsporozoite protein-encoding gene of Plasmodium vivax? Proc Natl Acad Sci USA 85:8102–8106
Arnot D (1989) Malaria and major histocompatibility complex. Parasitol Today 5:138–143
Conway DJ (1997) Natural selection on polymorphic malaria antigens and the search for a vaccine. Parasitol Today 13:26–29
Dover G (1982) Molecular drive: a cohesive mode of species evolution. Nature 299:111–117
Epping RJ, Goldstone SD, Ingram LT, Upcroft JA, Ramasamy R, Cooper JA, Bushell GR, Geysen HM (1988) An epitope recognised by inhibitory monoclonal antibodies that react with a 51 kilodalton merozoite surface antigen in Plasmodium falciparum. Mol Biochem Parasitol 28:1–10
Feiger I, Tavul L, Beck HP (1993) Plasmodium falciparum: a rapid technique for genotyping the merozoite surface protein 2. Exp Parasitol 77:372–375
Feiger I, Tavul L, Kabintik S, Marshall V, Genton B, Alpers M, Beck HP (1994) Plasmodium falciparum: extensive polymorphism in merozoite surface antigen 2 alleles in an area with endemic malaria in Papua New Guinea. Exp Parasitol 79:106–116
Foley M, Ranford-Cartwright LC, Babiker HA (1992) Rapid and simple method for isolating malaria DNA from fingerprick samples of blood. Mol Biochem Parasitol 53:241–244
Frontali C, Pizzi E (1991) Conservation and divergence of repeated structures in Plasmodium genomes: the molecular drift. Acta Leid 60(l):69–81
Frontali C (1994) Genome plasticity in Plasmodium. Genetica 94:91–100
Good MF, Pombo D, Quakyi IA, Riley EM, Houghten RA, Menon A, Ailing DW, Berzofsky JA, Miller LH (1988) Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum:immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci USA 85:1199–1203
Huang X (1994) On global sequence alignment. Computer Applications Biosci 10(3):227–235
Hughes AL (1991) Circumsporozoite protein genes of malaria parasites (Plasmodium spp.): evidence for positive selection on immunogenic regions. Genetics 127:345–353.
Hughes AL (1992) Positive selection and interallelic recombination at the merozoite surface antigen-1 (MSA-1) locus of Plasmodium falciparum. Mol Biol Evol 9:381–393
Hughes MK, Hughes AL (1995) Natural selection on Plasmodium surface proteins. Mol Biochem Paras 71:99–113
Li WH (1993) Unbiased estimation of the rate of synonymous and nonsynonymous substitution. J Mol Evol 36:96–99
Marshall VM, Anthony RL, Bangs MJ, Purnomo, Anders RF, Coppel RL (1994) Allelic variants of the Plasmodium falciparum merozoite surface antigen 2 (MSA-2) in a geographically restricted area of Irian Jaya. Mol Biochem Parasiol 63(1): 13–21
Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T (1989) Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci USA 86:2766–2770
Pizzi E, Liuni S, Frontali C (1990) Detection of latent sequence periodicities. Nucl Acid Res 18(3): 3745–3752
Prescott N, Stowers AW, Cheng Q, Bobogare A, Rzepczyk CM, Saul A (1994) Plasmodium falciparum genetic diversity can be characterised using the polymorphic merozoite surface antigen 2 (MSA-2) gene as a single locus marker. Mol Biochem Paras 63:203–212
Rzepczyk CM, Csurhes PA, Lord R, Matile H (1990) Synthetic peptide immunogens eliciting antibodies to Plasmodium falciparum sporozoite and merozoite surface antigens in H 2b and H 2k mice. J Immunol 145:2691–2696
Rzepczyk CR, Csurhes PA, Saul AJ, Jones GL, Dyer S, Chee D, Goss N, Irving DO (1992) Comparative study of the T cell response to two allelic forms of a malarial vaccine candidate protein. J Immunol 148:1197–1204
Saul A, Battistutta D (1988) Codon usage in Plasmodium falciparum. Mol Biochem Parasitai 27:35–42
Smythe JA, Coppel RL, Brown GV, Ramasamy R, Kemp DJ, Anders RF (1988) Identification of two integral membrane proteins of Plasmodium falciparum. Proc Natl Acad Sci USA 85:5195–5199
Smythe JA, Peterson MG, Coppel RL, Saul AJ, Kemp DJ, Anders RF (1990) Structural diversity in the 45 kilodalton merozoite surface antigen of Plasmodium falciparum. Mol Biochem Parasitol 39(2): 227–234
Smythe JA, Coppel RL, Day KP, Martin RK, Oduola AMJ, Kemp DJ, Anders RF (1991) Structural diversity in the Plasmodium falciparum merozoite surface antigen 2. Proc Natl Acad Sci USA 88: 1751–1755
Snewin VA, Herrera M, Sanchez G, Scherf A, Langsley G, Herrera S (1991) Polymorphism of the alleles of the merozoite surface antigens MSA1 and MSA2 in Plasmodium falciparum wild isolates from Colombia. Mol Biochem Parasitol 49:265–276
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Felger, I., Marshal, V.M., Reeder, J.C. et al. Sequence diversity and molecular evolution of the merozoite surface antigen 2 of plasmodium falciparum . J Mol Evol 45, 154–160 (1997). https://doi.org/10.1007/PL00006215
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/PL00006215