Abstract.
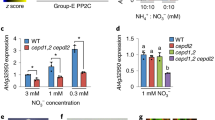
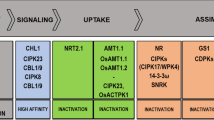
This review highlights progress in dissecting how plant nitrate reductase (NR) activity is regulated by Ca2+, protein kinases, protein kinase kinases, protein phosphatases, 14-3-3 proteins and protease(s). The signalling components that regulate NR have also been discovered to target other enzymes of metabolism, vesicle trafficking and cellular signalling. Extracellular sugars exert a major impact on the 14-3-3-binding status and stability of many target proteins, including NR in plants, whereas other stimuli affect the regulation of some targets and not others. We thus begin to see how selective or global switches in cellular behaviour are triggered by regulatory networks in response to different environmental stimuli. Surprisingly, the question of how changes in NR activity actually affect the rate of nitrate assimilation is turning out to be a tough problem.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MacKintosh*, C., Meek, S. Regulation of plant NR activity by reversible phosphorylation, 14-3-3 proteins and proteolysis . CMLS, Cell. Mol. Life Sci. 58, 205–214 (2001). https://doi.org/10.1007/PL00000848
Issue Date:
DOI: https://doi.org/10.1007/PL00000848