Abstract
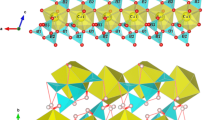
The powder EPR spectra of portlandite (Ca(OH)2), brucite (Mg(OH)2) and hydrotalcite ([Mg3Al(OH)8]+[0.5CO -3 ·nH2O]-), doped with 63CU2+, have been measured as a function of temperature. In all materials a static, temperature independent Jahn-Teller site and a dynamic, temperature dependent Jahn-Teller site was observed. In the rapidly reorienting situation the EPR spectrum of the dynamic site is still anisotropic. From the observed anisotropy it could be inferred that the hydroxyde ions surrounding the Cu2+ ion are situated at the vertices of an elongated octahedron which is compressed along its trigonal axis (c-axis). The angle between the axis of elongation of the Cu octahedra and the c-axis turned out to be 490 both for portlandite and brucite. The temperature range over which the averaged dynamic site could be observed varied considerably for the three materials.
Similar content being viewed by others
References
Chibwe K., Jones W.: J. Chem. Soc. Chem. Commun. 1989, 926.
Tanaka M., Park I.Y., Kuroda K., Kato C.: Bull. Chem. Soc. Jpn. 62, 3442 (1989)
Pinnavaia T.J., Giannelis E.P., Nozera D.G.: Inorg. Chem. 26, 203 (1987)
Allmann R., Jepsen H: Neues Jb. Min. Mh. 1969, 544.
Suzuki E., Okamoto M., Ono Y.: Chemistry Letters 1989, 1485.
Union Carbide: Catalyzed Aldol Condensations, EP 95783 A2 (1983)
Reichle W.T.: U.S. 4, 458, 026, To Union Carbide Comp., July 3 (1984)
Reichle W.T.: J. of Catalysis 94, 547 (1985)
Kikkawa S., Koizumi M.: Mat. Res. Bull. 17, 191 (1982)
Schöllhorn R., Otto B.: J. Chem. Soc. Chem. Commun. 1986, 1222.
Schutz A., Bileon P.: J. Solid State Chem. 68, 360 (1987)
Yasunaga T., Ikeda T., Amoh H.: J. Am. Chem. Soc. 106, 5772 (1984)
Yasunaga T., Mikami N., Sasaki M., Horibe S.: J. Phys. Chem. 88, 1716 (1984)
Bish DL: Bull. Mineral. 103, 170 (1980)
Brindley G.W., Kikkawa S.: Clays Clay Miner. 28, 87 (1980)
Mendiboure A., Schöllhorn R: Rev. Chim. Miner. 23, 819 (1986)
LeBail C., Thomassin J.H., Touray J.C.: Phys. Chem. Minerals 14, 377 (1987)
Miyata S., Okada A.: Clays Clay Miner. 25, 14 (1977)
Sato T., Wakabayashi T., Shimada M.: Ind. Eng. Chem. Prod. Res. Dev. 25, 89 (1986)
Wilson RG., Holuj F., Hedgecock N.E.: Phys. Rev. B. 1, 3609 (1970)
Holuj F., Wilson R.G.: Phys. Rev. B. 7, 4065 (1973)
Keijzers C.P., Reijerse E.J., Stam P., Dumont M.F., Gribnau M.C.M.: J. Chem. Soc. Far. Trans. 183, 3493 (1987)
Philips V.A., Kolbe J.L., Opperhauser H.: Prac. Intern. Conf. on Colloids and Surlaces 4, 169 (1976)
Philips V.A., Kolbe JL, Opperhauser H.: J. Cryst. Growth J. Cryst. Growth, 228 (1977)
Packter A.: Crystal Research & Technol. 20, 329 (1985)
Philips V.A., Kolbe J.L., Opperhauser H.: J. Cryst. Growth 41, 235 (1977)
Tagai H., Saito K.: Yogyo Kyokai Shi 76, 81 (1968)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Pol, A., Peters, G., Reijerse, E. et al. An EPR Study of Cu2+ Doped Portlandite, Brucite and Hydrotalcite. Appl Magn Reson 3, 751–762 (1992). https://doi.org/10.1007/BF03356738
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03356738