Abstract
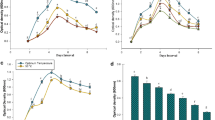
The objective of this study was to screen the potential of four plant growth promoting rhizobacteria (PGPR) for growth promotion in sorghum (Sorghum bicolor) and suppression of Striga hermonthica development. Bacillus subtilis Bsn5, B. subtilis GBO3, B. amyloliquefaciens FZB42 and Burkholderia phytofirmans PsJN were evaluated under controlled conditions in growth chambers. After 28 days of growth, the effect of selected PGPR on sorghum plant height, leaf chlorophyll (SPAD) value, biomass dry matter (DM), number of germinated and attached Striga plants and number of Striga plants that developed tubercles was analyzed. Inoculated Striga-free sorghum plants were significantly taller, with higher leaf chlorophyll SPAD values and higher dry matter than uninoculated Striga-free plants. However, there were no differences in sorghum height, SPAD value and DM between inoculated and uninoculated Striga-infected sorghum plants. In the absence of PGPR inoculation, differences in DM were observed between Striga-free and Striga-infected sorghum. Compared to the untreated control, Striga seed germination was lower in the sorghum plants treated with B. subtilis GBO3 and B. amyloliquefaciens FZB42. Of the germinated Striga seeds, the percentage that attached to the sorghum plant was lowest (23%) in the B. subtilis GBO3 treatment. The percentage of dead Striga tubercles in the PGPR treatments ranged between 35 and 59%, compared to 3% in the untreated control. This study identified B. subtilis GBO3, B. amyloliquefaciens FZB42 and Burkholderia phytofirmans PsJN with promising potential to promote sorghum growth and suppress Striga.
Similar content being viewed by others
References
Adesemoye A, Torbert H & Kloepper J, 2008. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can J Microbiol 54, 876–886.
Ait Barka E, Sabine GS, Nowak J, Jean-Claude A & Belarbi A, 2002. Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol Control 24, 135–142.
Amusan IO, Rich PJ, Menkir A, Housley T & Ejeta G, 2008. Resistance to Striga hermonthica in a maize inbred line derived from Zea diploperennis. New Phytol 178, 157–166.
Amusan IO, Rich PJ, Housley T & Ejeta G, 2011. An in vitro method for identifying post attachment Striga resistance in maize and sorghum. Agron J 103, 1472–1478.
Arnaud MC & Véronési C & Thalouarn P, 1999. Physiology and histology of resistance to Striga hermonthica in Sorghum bicolor var. Framida. Aust J Plant Physiol 26, 63–70.
Babalola OO, 2010. Beneficial bacteria of agricultural importance. Biotechnol Lett 32, 1559–1570.
Deng Y, Zhu Y, Wang P, Zhu L, Zheng J, Li R & Sun M, 2011.Complete genome sequence of Bacillus subtilis BSn5, an endophytic bacterium of Amorphophallus konjac with antimicrobial activity for the plant pathogen Erwinia carotovora subsp. carotovora. J Bacteriol 193, 2070–2071.
FAO, 2012 statistical databases. http://faostat.fao.org. (Last accessed 20 April 2014).
Frommel MI, Nowak J & Lazarovits G, 1991. Growth enhancement and developmental modifications of in vitro grown potato (Solanum tuberosum spp. tuberosum) as affected by a nonfluorescent Pseudomonas sp. Plant Physiol 96, 928–936.
Glick BR, 1995. The enhancement of plant growth by free-living bacteria. Can J Microbiol 41, 109–117.
Goldwasser Y, Hershenhorn J, Plakhine D, Kleifeld Y & Rubin B, 1999. Biochemical factors involved in Vetch resistance to Orobanche aegyptiaca. Physiol Mol Plant Pathol 54, 87–96.
Graves JD, Press MC & Stewart GR, 1989. A carbon balance model of sorghum-Striga host-parasite association. Plant Cell Environ 12, 101–107.
Grosch R, Junge H, Krebs B & Bochow H, 1999. Use ofBacillus subtilis as a biocontrol agent. III. Influence of Bacillus subtilis on fungal root diseases and on yield in soilless culture. J Plant Dis Protect 106, 568–580.
Guo C, Cui W, Feng X, Zhao J & Lu G, 2011. Sorghum insect problems and management. J Integr Plant Biol 53, 178–192.
Haussmann BIG, Hess DE, Reddy BVS, Mukuru SZ, Kayentao M, Welz HG & Geiger HH, 2000. Diallel studies on Striga resistance in sorghum. In: Haussmann, BIG, Koyama, ML, Grivet, L, Rattunde, HF, Hess, DE (Eds.) 2000: Proceedings of a Workshop on Breeding for Striga Resistance in Cereals. IITA, Ibadan, Nigeria, 18–20 August 1999. Margraf, Weikersheim, Germany. 29–37.
Idris HA, Labuschagne N & Korsten L, 2008. Suppression of Pythium ultimum root rot of sorghum by rhizobacterial isolates from Ethiopia and South Africa. Biol Control 45, 72–84.
Jamil M, van Mourik TA, Charnikhova T & Bouwmeester HJ, 2013. Effect of diammonium phosphate on strigolactone production and Striga hermonthica infection in three sorghum cultivars. Weed Res 53, 121–130.
Kim S, Lowman S, Hou G, Nowak J, Flinn B & Mei C, 2012. Growth promotion and colonization of switchgrass (Panicum virgatum) cv. Alamo by bacterial endophyte Burkholderia phytofirmans strain PsJN. Biotechnol Biofuels 5, 1–10.
Lazarovits G & Nowak J, 1997. Rhizobacteria for improvement of plant growth and establishment. HortScience 32, 188–192.
Pedas P, Hebbern CA, Schjoerring JK, Holm PE & Husted S, 2005. Differential capacity for high-affinity manganese uptake contributes to differences between barley genotypes in tolerance to low manganese availability. Plant Physiol 139, 1411–1420.
Press MC & Stewart GR, 1987. Growth and photosynthesis in Sorghum bicolor infected with Striga hermonthica. Ann Bot 60, 657–662.
Runo S, Macharia S, Alakonya A, Machuka J, Sinha N & Scholes J, 2012. Striga parasitizes transgenic hairy roots of Zea mays and provides a tool for studying plant-plant interactions. Plant Methods 8, 1–11.
Sauerborn J, 1991. The economic importance of the phytopa-rasits Orobanche and Striga. In: Ransom, JK, Musselman, LJ, Worsham, D and Parker, C (Eds.) 1991: Proceedings of the 5th International Symposium on Parasitic Weeds. CIMMYT, Nairobi, Kenya. 137–143.
Sauerborn J & Müller-Stöver D, 2009. Application of natural antagonists including arthropods to resist weedy Striga (Orobanchaceae) in tropical agroecosystems. In: Muniappan, R, Reddy, GVP, Raman, A (Eds.) 2009: Biological Control of Tropical Weeds using Arthropods. Cambridge University Press, Cambridge, UK. 423–433.
Scholes JD & Press MC, 2008. Striga infestation of cereal crops — an unsolvedproblem in resource limited agriculture. Curr Opin Plant Biol 11, 180–186.
Sessitsch A, Coenye T, Sturz AV, Vandamme P, Barka EA, Salles JF & van Elsas JD, 2005. Burkholderia phytofirmans spp. Nov., a novel plant-associated bacterium with plant-beneficial properties. Int J Syst Evol Micr 55, 1187–1192.
Těšitel J, Plavcova L & Cameron D, 2010. Heterotrophic carbon gain by the root hemiparasites, Rhinanthus minor and Euphrasia rostkoviana (Orobanchaceae). Planta 231, 1137–1144.
Watson AK, 2013. Biocontrol. In: Joel, DM., Gressel, J., Muselman, LJ. (Eds.) 2013: Parasitic Orobancheceae: Parasitic Mechanisms and Control Strategies. Springer-Verlag Berlin Heidelberg. 469–497.
Zhang H, Kim M, Krishnamachari V, Payton P, Sun Y, Grimson M & Melo IS, 2007. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226, 839–851.
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S & Paré PW, 2008. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 56, 264–273.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mounde, L.G., Boh, M.Y., Cotter, M. et al. Potential of Rhizobacteria for Promoting Sorghum Growth and Suppressing Striga hermonthica Development. J Plant Dis Prot 122, 100–106 (2015). https://doi.org/10.1007/BF03356537
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03356537