Abstract
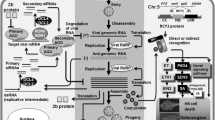
The Turnip Crinkle Virus (TCV)–Arabidopsis interaction is one of the best-characterized pathosystems, and studies of this system have contributed significantly to the understanding of plant resistance to viral pathogens. Inoculation of TCV on the resistant Arabidopsis ecotype Di-17 elicits the hypersensitive response (HR), and is accompanied by increased expression of defense genes. The HR to TCV infection is conferred by HRT (HR to TCV) gene, which encodes a CC-NBS-LRR class resistance (R) protein. In contrast to the HR, resistance requires the HRT gene and a recessive locus designated rrt. Unlike most CC-NBS-LRR R proteins, HRT-mediated resistance is dependent on EDS1 (enhanced disease susceptibility 1) and is independent of NDR1 (non-race-specific disease resistance). Resistance is also independent of RAR1 (required for Mla12 resistance), SGT1 (suppressor of the G2 allele of SKP1), and TCV-interacting protein (TIP) but is compromised in the salicylic acid (SA) deficient mutants eds5 (enhanced disease susceptibility 5), pad4 (phytoalexin deficient 4), sid2 (salicylic acid induction deficient 2), and sag101 (senescence associated gene 101). EDS1 and SA have redundant functions in the induction of the HR by HRT. SAG101 interacts with PAD4, via EDS1, and EDS1, PAD4, and SAG101 form a ternary complex and perform independent functions in HRT-mediated resistance. HRT interacts with CRT1 (compromised recognition of TCV), an ATPase and resistance to TCV is partially compromised by a mutation in CRT1. HRT-mediated HR and TCV resistance are also dependent on light. A dark treatment, immediately following TCV inoculation, suppresses the HR, TCV resistance, and the activation of a majority of the TCV-in-duced genes. Interestingly, a mutation in blue-light photo-receptors compromised TCV resistance and led to degradation of HRT via a proteasome-dependent pathway, resulting in susceptibility to TCV, which correlates with its interaction with the E3 ubiquitin ligase, COP1 (constitutively photo-morphogenesis 1). This review aims to document the advances in the understanding of the TCV-Arabidopsis patho-system as a case study and provide a valuable model for dissecting plant resistance to viral pathogens.
Similar content being viewed by others
Abbreviations
- CC-NBS-LRR:
-
Coiled-coil nucleotide-binding site leucine-rich repeat
- COP1:
-
Constitutively photomorphogenesis 1
- CP:
-
Coat protein
- CRT1:
-
Compromised recognition of TCV
- CRY:
-
Cryptochrome
- EDS1:
-
Enhanced Disease Susceptibility 1
- EDS5:
-
Enhanced Disease Susceptibility 5
- ET:
-
Ethylene
- HR:
-
Hypersensitive response
- HRT:
-
HR to TCV
- JA:
-
Jasmonic acid
- NPR1:
-
Nonexpressor of PR
- PAD4:
-
Phytoalexin Deficient 4
- PAL:
-
Phenylalanine ammonia lyase
- PHOT:
-
Phototropin
- PHY:
-
Phytochrome
- PR:
-
Pathogenesis-related
- PTI:
-
PAMP-triggered immunity
- R:
-
Resistance
- SA:
-
Salicylic acid
- SAG101:
-
Senescence Associated Gene 101
- SAR:
-
Systemic acquired resistance
- SID2:
-
Salicylic acid Induction Deficient 2
- TCV:
-
Turnip Crinkle Virus
- TIP:
-
TCV interacting protein
References
Briggs WR & Huala E, 1999. Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol 15, 33–62.
Carrington JC, Heaton LA, Zuidema D, Hillman BI & Morris JT, 1989. The genome structure of turnip crinkle virus. Virology 170, 219–226.
Cashmore AR, 2003. Cryptochromes: Enabling plants and animals to determine circadian time. Cell 114, 12142–12147.
Century KS, Holub EB & Staskawicz BJ, 1995. NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc Natl Acad Sci USA 92, 6597–6601.
Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Klessig D, Kachroo A & Kachroo P, 2006. Light-dependent hypersensitive response and resistance signaling against Turnip Crinkle Virus in Arabidopsis. Plant J 45, 320–334.
Chandra-Shekara AC, Navarre D, Kachroo A, Kang HG, Klessig D & Kachroo P, 2004. Signaling requirements and role of salicylic acid in HRT- and rrt-mediated resistance to Turnip Crinkle virus in Arabidopsis. Plant J 5, 647–659.
Chandra-Shekara AC, Venugopal SC, Barman SR, Kachroo A & Kachroo P, 2007. Plastidial fatty acid levels regulate resistance gene-dependent defense signaling in Arabidopsis. Proc Natl Sci USA 104, 7277–7282.
Christie JM, 2007. Phototropin blue-light receptors. Annu Rev Plant Biol 58, 21–45.
Dangle JL & Jones JD, 2001. Defense responses to infection. Nature 411, 826–833.
Dempsey DA, Wobbe KK & Klessig DF, 1993. Resistance and susceptible responses of Arabidopsis thaliana to Turnip Crinkle virus. Phytopathology 83, 1021–1029.
Feys BJ, Moisan LJ, Newman MA & Parker J, 2001. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 20, 5400–5411.
Feys BJ, Wiermer M, Bhat RA, Moisan L, Medina-Escobar N, Neu C, Cabral A & Parker J, 2005. Arabidopsis SENESCENCE-ASSOCIATED GENE 101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17, 2601–2613.
Flor H, 1971. Current status of gene-for-gene concept. Annu Rev Phytopathol 9, 275–296.
Garcia AV, Blanvillain-Baufume S, Huibers RP, Wiermer M, Li G, Gobbato E, Rietz S & Parker J, 2010. Balanced nuclear and cytoplasmic activities of EDS1 are required for a complete plant innate immune response. PLoS Pathog 6, e1000970.
Griebel T & Zeier J, 2008. Light regulation and day-time dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol 147, 790–801.
Jeong RD, Chandra-Shekara AC, Barman SR, Navarre DA, Klessig D, Kachroo A & Kachroo P, 2010a. CRYPTO-CHROME 2 and PHOTOTROPIN 2 regulate resistance protein mediated viral defense by negatively regulating an E3 ubiquitin ligase. Proc Natl Acad Sci USA 107, 13538–13543.
Jeong RD, Kachroo A & Kachroo P, 2010b. Blue light photo-receptors are required for the stability and function of a resistance protein mediating viral defense in Arabidopsis. Plant Signaling Behav 5, 1504–1509.
Jeong RD, Chandra-Shekara AC, Kachroo P, Klessig DF & Kachroo P, 2008. HRT-mediated hypersensitive response and resistance to Turnip Crinkle Virus in Arabidopsis does not require the function of TIP, the presumed guardee protein. Mol Plant-Microbe Interact 21, 1316–1324.
Jia Y, Bryan G, McAdams, Bryan GT, Hershey HP & Valent B, 2000. Direct interaction of resistance gene and avirulence gene products confers rice resistance. EMBO J 19, 4004–4014.
Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM & Glazebrook J, 1999. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci USA 96, 13583–13588.
Kachroo P, Chandra-Shekara AC & Klessig DF, 2006. Plant signal transduction and defense against viral pathogens. Adv Virus Res 60, 161–191.
Kachroo P, Yoshioka K, Shah J, Dooner HK & Klessig DF, 2000. Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes, is salicylic acid dependent but NPR1, ethylene and jasmonate independent. Plant Cell 12, 677–690.
Kang HG, Hyong WC, von Einem S, Manosalva P, Ehlers K, Liu PP, Buxa SV, Moreau M, Mang HG, Kachroo P, Kogel KH & Klessig DF, 2012. CRT1 is a nuclear-translocated MORC endonuclease that participates in multiple levels of plant immunity. Nature Commun 3, 1297.
Kang HG, Kuhl JC, Kachroo P & Klessig DF, 2008. CRT1, an Arabidopsis ATPase that interacts with diverse resistance proteins and modulates disease resistance to turnip crinkle virus. Cell Host Microbe 17, 48–57.
Kang HG, Oh CS, Sato M, Katagiri F, Glazebrook J, Takahashi H, Kachroo P, Martin GB & Klessig DF, 2010. Endosome-as-sociated CRT1 functions early in resistance gene-mediated defense signaling in Arabidopsis and tobacco. Plant Cell 22, 918–936.
Karpinski S, Gabrys H, Mateo A, Karpinska B & Mullineaux P, 2003. Light perception in plant disease defense signaling. Curr Opin Plant Biol 6, 390–396.
Kohm BA, Goulden MG, Gilbert JE, Kavanagh TA & Baulcombe DC, 1993. A Potato Virus X resistance gene mediates an induced, nonspecific resistance in protoplasts. Plant Cell 8, 913–920.
Kunkel BN & Brooks DM, 2002. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5, 325–331.
Lau OS & Deng XW, 2012. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17, 584–593.
Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, Llorente F, Molina A, Parker J, Somerville S & Schulze-Lefert P, 2005. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310, 1180–1183.
Liu H, Liu B, Zhao C, Pepper M & Lin C, 2011. The action mechanisms of plant cryptochromes. Trends Plant Sci 16, 684–691.
Martin GB, Bogdanove AJ & Sessa G, 2003. Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol 54, 23–61.
Mauch-Mani B & Slusarenko A, 1996. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 8, 203–212.
Nawrath C, Heck S, Parinthawong N & Metraux JP, 2002. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14, 275–286.
Nimchuk Z, Marois E, Kjemtrup S, Leister RT, Katagiri F & Dangle JL, 2000. Eukaryotic fatty acylation drives plasma membrane targeting and enhances function of several type III effector proteins from Pseudomonas syringae. Cell 101, 353–363.
Parker JE, Holub EB, Frost LN, Falk A, Gunn ND & Daniels MJ, 1996. Characterization of eds1, a mutation in Arabi-dopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8, 2033–2046.
Ren T, Qu F & Morris TJ, 2005. The nuclear localization of the Arabidopsis transcription factor TIP is blocked by its interaction with the coat protein of Turnip crinkle virus. Virology 331, 316–324.
Sakamoto K & Briggs W, 2000. Cellular and subcellular localization of phototropin 1. Plant Cell 14, 1723–1735.
Soosaar JL, Burch-Smith TM & Dinesh-Kumar SP, 2005. Mechanisms of plant resistance to viruses. Nat Rev Microbiol 3, 789–798.
Takahashi H, Miller J, Nozaki Y, Takeda M, Shah J, Hase J, Ikegami M, Ehara Y & Dinesh-Kumar SP, 2002. RCY1, an Arabidopsis thaliana RPP8/HRT family resistance gene, conferring resistance to cucumber mosaic virus requires salicylic acid, ethylene and a novel signal transduction mechanism. Plant J 32, 655–667.
Takahashi H, Kanayama Y, Zheng MS, Kusano T, Hase S, Ikegami M & Shah J, 2004. Antagonistic interactions between the SA and JA signaling pathways in Arabidopsis modulate expression of defense genes and gene-for-gene resistance to cucumber mosaic virus. Plant Cell Physiol 45, 803–809.
Tang X, Frederick RD, Zhou J, Halterman DA, Jia Y & Martin GB, 1996. Initiation of plant disease resistance by physical interaction of AvrPto and Ptokinase. Science 274, 2060–2063.
Van der Biezen EA & Jones JD, 1998. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci 23, 454–456.
Venugopal SC, Jeong RD, Mandal MK, Zhu S, Chandra AC, Xia Y, Hersh M, Stromberg AJ, Navarre D, Kachroo A & Kachroo P, 2009. Enhanced disease susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signaling. PLoS Genetics 5, e1000545.
Wiermer M, Feys BJ & Parker JE, 2005. Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol 8, 383–389.
Wildermuth MC, Dewdney J, Wu G & Ausubel FM, 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 414, 562–565.
Wu L & Yang HQ, 2010. CRYPTOCHROME 1 is implicated in promoting R protein-mediated plant resistance to Pseudo-monas syringae in Arabidopsis. Mol Plant 3, 539–548.
Zaitlin M & Palukaitis P, 2000. Advances in understanding plant viruses and virus diseases. Annu Rev Phytopathol 38, 117–143.
Zhu S, Jeong RD, Venugopal SC, Lapchyk L, Navarre D, Kachroo A & Kachroo P, 2011. SAG101 forms a ternary complex with EDS1 and PAD4 and is required for resistance signaling against Turnip Crinkle Virus. PLoS Pathog 7, e1002318.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, MA., Jeong, RD. Resistance protein-mediated defense signalling in response to Turnip Crinkle Virus in Arabidopsis: recent advances. J Plant Dis Prot 120, 97–104 (2013). https://doi.org/10.1007/BF03356460
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03356460