Abstract
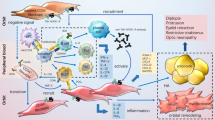
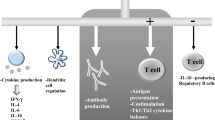
We have studied a possible role of T cell sensitization to eye muscle antigens in patients with thyroid-associated ophthalmopathy (TAO). Peripheral blood mononuclear cell (PBMC) proliferation in response to crude porcine orbital tissue antigens, partially purified porcine eye muscle membrane proteins and predicted epitopic fragments of the recombinant 64 kDa protein 1D, was determined in patients with TAO and thyroid autoimmunity without eye disease. When membrane and cytosol fractions were used as antigen PBMC from 43% of patients with TAO but only 12.5% of normal subjects were responsive to a crude orbital connective tissue membrane fraction, although this difference was not significant. We were unable to demonstrate specific recognition of partially purified eye muscle membrane fractions; although most of the fractions tested were occasionally recognized by T cells from patients with ophthalmopathy, this was also the case for patients with autoimmune thyroid disease without ophthalmopathy and normal subjects. We did not clearly identify epitopic sequences within the 1D protein, most of the predicted peptides tested being recognized not only by T cells from a small proportion of patients with TAO, but also by those from some patients with autoimmune thyroid disease without ophthalmopathy and normal subjects. It is noteworthy however that approximately 22% of TAO patients, but no normal subjects, were positive to one or more of three peptides, suggesting that reactivity to the 1D protein may play a role in the pathogenesis of the eye disorder in some patients with TAO. The inconsistent and generally low T cell responses to crude and purified antigens noted in a few patients with TAO could be explained by low numbers of specifically sensitized lymphocytes in peripheral blood.
Similar content being viewed by others
References
Burch H.B., Wartofsky L. Graves’ Ophthalmopathy: Current Concepts Regarding Pathogenesis and Management. Endoer. Rev. 14: 747, 1993.
Kiljanski J., Nebes V., Wall J.R. Is the ocular muscle a target of the immune system in endocrine ophthalmopathy. Pro. Intl. Arch. Allergy Immunol., 106: 204, 1995.
Salvi M., Miller A., Wall J.R. Human orbital tissue and thyroid membranes express a 64 kDa protein which is recognized by autoantibodies in the serum of patients with thyroid-associated ophthalmopathy. FEBS Lett. 232: 135, 1988.
Miller A., Arthurs B., Boucher A., Liberman A., Bernard N., Rodien P., Salvi M., Wall J.R. Significance of antibodies reactive with a 64 kDa eye muscle membrane antigen in patients with thyroid autoimmunity. Thyroid 2: 197, 1992.
Salvi M., Bernard N., Miller A., Zhang Z.G., Gardini E., Wall J.R. Prevalence of antibodies reactive with a 64 kDa eye muscle membrane antigen in thyroid-associated ophthalmopathy. Thyroid 1: 204, 1991.
Wall J.R., Bernard N., Boucher A., Salvi M., Zhang Z.G., Kennerdell J., Tyutyunikov A., Genovese C. Pathogenesis of thyroid-associated ophthalmopathy: An autoimmune disorder of the eye muscle associated with Graves’ hyperthyroidism and Hashimoto’s thyroiditis. Clin. Immunol. Immunopathol. 68: 1, 1993.
Dong Q., Ludgate M., Vassart G. Cloning and sequencing of a novel 64 kDa autoantigen recognized by patients with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 92: 1375, 1991.
Bernard N.F., Salvi M., Tyutyunikov A., Genovese C., Ludgate M., Wall J.R. Antibodies against the recombinant 64 kDa membrane protein 1D are associated with ophthalmopathy in patients with thyroid autoimmunity. Clin. Immunol. Immunopath. 70: 225, 1994.
Cornette J.L, Margalit H., DeLisi C, Berzofsky J.A. Identification of T-cell epitopes and use in construction of synthetic vaccine. Methods in Enzymology 178: 611, 1989.
Berzofsky J.A. Mechanisms of T cell recognition with application to vaccine design. Mol. Immunol. 28: 217, 1991.
Margalit H., Spouge J.L., Cornette K., Cease K., DeLisi C, Berzofsky J.A. Prediction of immunodominant helper T-cell antigenic sites from the primary sequence. J. Immunol. 138: 2213, 1987.
Committees of Regional Thyroid Association. Classification of eye changes of Graves’ disease. Thyroid 2: 235, 1992.
Wall J.R., Strakosch CR., Fang S.L., Ingbar S.H., Braverman L. Thyroid binding antibodies and other immunological abnormalities in patients with Graves’ ophthalmopathy: Effect of treatment with cyclophosphamide. Clin. Endocrinol. 10: 79, 1979.
Laemmli U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680, 1970.
Wall J.R. Nature and significance of eye muscle autoantigens in endocrine ophthalmopathy. In: Kahaly G. (Ed), Endocrine Ophthalmopathy. Molecular, Immunological and Clinical Aspects. Dev. Ophthalmol. Basel, Karger, 25, 1993, p. 77.
Tallstedt L, Norberg R. Immunohistochemical staining of normal and Graves’ extraocular muscle. Vestigative Ophthalmology and Visual Science 29: 175, 1988.
Weetman A.P., Cohen S., Gatter K.C., Fells P., Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves’ ophthalmopathy. Clin. Exp. Immunology 75: 222, 1989.
Bahn R.S., Heufelder A.E. Orbital connective tissue in endocrine ophthalmopathy. In: Kahaly G. (Ed.), Endocrine Ophthalmopathy. Molecular, Immunological and Clinical Aspects. Dev. Ophthalmol. Karger, Basel, 25: 46, 1993.
Hiromatsu Y., Fukazawa H., How J., Wall J.R. Antibody-dependent cell-mediated cytotoxicity against human eye muscle cells and orbital fibroblasts in Graves’ ophthalmopathy — roles of Class II MHC antigen expression and n-interferon action on effector and target cells. Clin. Exp. Immunol. 70: 593, 1987.
Hiromatsu Y., Tanaka K., Ishisaka N., Nonaka K., Hoshino T., Inoue Y. HLA-DR antigen expression on eye muscle in thyroid-associated ophthalmopathy. In: Nagataki S., Mori T., Torizuka K. (Eds.), 80 years of Hashimoto Disease. Excerpta Medica Elsevier Science Publishers B.V., 1993, p. 349.
Rosen C, Raikow R.B., Bürde R.M., Kennerdell J.S., Mosseri M., Scalise D. Immunohistochemical evidence for IgGAI involvement in Graves’ ophthalmopathy. Ophthalmol. 99: 146, 1992.
Arnold K., Tandon N., Macintosh R.S., Ludgate M., Weetman A.P. T cell responses to orbital antigens inn thyroid-associated ophthalmopathy. Clin. Exp. Immunol. 96: 329, 1994.
Wall J.R., Scalise D., Hayes M., Stolarski C, Sato M., Salvi M., Nebes V. Native gel electrophoresis of the 64 kDa protein shows that is eye muscle specific and an important target for autoantibodies in patients with thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 80: 1226, 1995.
Ross P.V., Koenjg R.J., Arscott P., Ludgate M., Waier M., Nelson C.C., Kaplan M., Baker J.R. Tissue specificity and serologic reactivity of an autoantigen associated with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 77: 433, 1993.
Tandon N., Weetman A.P.O. T cells and thyroid autoimmunity. J. R. Coll. Physicians Lond. 28: 10, 1994.
Arnold K., Weetman A.P. Cell-mediated immunity in thyroid-associated ophthalmopathy. Orbit (In press).
McLachlan S.M., Proud G., Pegg C.A., Clark F., Rees-Smith B. Functional analysis of T and B cells from blood and thyroid tissue in Hashimoto’s disease. Clin. Exp. Immunol. 59: 585, 1985.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kiljanski, J., Stolarski, C., Barsouk, A. et al. Failure to demonstrate cell-mediated immunity to orbital tissue antigens and epitopic fragments of a 64 kDa protein in the majority of patients with thyroid-associated ophthalmopathy. J Endocrinol Invest 19, 284–292 (1996). https://doi.org/10.1007/BF03347864
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03347864