Abstract
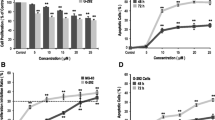
The key pharmacological action for the clinical use of bisphosphonates lies in the inhibition of osteoclast-mediated bone resorption. Osteoblasts could be other target cells for bisphosphonates. We studied the effects of bisphosphonates on the proliferation and the differentiation of normal human bone trabecular osteoblastic cells (hOB). We tested 4 different compounds: clodronate, pamidronate and 2 newer compounds: ibandronate, a nitrogen-containing bisphosphonate and zoledronate, which is a heterocyclic imidazole compound. Ibandronate and zoledronate stimulated hOB cell proliferation by up to 30% (p<0.05) after 72 h for concentrations ranging from 10−8 M to 10−5 M. Clodronate transiently enhanced hOB cell survival after only 24 h (+60%, p<0.001) whereas pamidronate had no effect. Longer time course studies, in presence of fetal calf serum, revealed that cell growth was finally reduced by all 4 bisphosphonates (40% after 7 days). Type I collagen synthesis was transiently increased by all 4 bisphosphonates after only 48 h incubation (+17% to +67%, p<0.05). Clodronate increased ALP activity by up to 1.7-fold after 4 days of culture (p<0.05) whereas ibandronate or zoledronate exhibited lesser stimulatory effects (+17 to +30%), and pamidronate had no significant effect. In conclusion, we found that different bisphosphonates, currently used or tested in various clinical conditions, transiently stimulated the growth of preosteoblastic cells and thereafter increased their differentiation according to sequential events (type I collagen synthesis first, then ALP activity to a lesser extent). Our data suggest that the beneficial effects of bisphosphonate treatment on bone mass and integrity could be partly mediated through a direct action on osteoblasts.
Similar content being viewed by others
References
Lin J.H. Bisphosphonates: a review of their pharmacokinetic properties. Bone 1996, 18: 75–85.
Masarachia P., Weinreb M., Balena R., Rodan G.A. Comparison of the distribution of 3H-alendronate and 3Hetidronate in rat and mouse bones. Bone 1996, 19: 281–290.
Sahni M., Guenther H.L., Fleisch H., Collin P., Martin T.J. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J. Clin. Invest. 1993, 91: 2004–2011.
Evans C.E., Braidman I.P. Effects of two novel bisphosphonates on bone cells in vitro. Bone Miner. 1994, 26: 95–107.
Hughes D.E., MacDonald B.R., Russell R.G., Gowen M.J. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J. Clin. Invest. 1989, 83: 1930–1935.
Hughes D.E., Wright K.R., Uy H.L., et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995, 10: 1478–1487.
Nishikawa M., Akatsu T., Katayama Y., et al. Bisphosphonates act on osteoblastic cells and inhibit osteoclast formation in mouse marrow cultures. Bone 1996, 18: 9–14.
Fleisch H. Bisphosphonates in bone disease. From the laboratory to the patient, ed. 3. Parthenon Publishing Group, New York, 1997.
Liberman U.A., Weiss S.R., Bröll J., et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study. N. Engl. J. Med. 1995, 333: 1437–1443.
Body J.J., Bartl R., Burckhardt P., et al. Current use of bisphosphonates in oncology. International bone and cancer study group. J. Clin. Oncol. 1988, 16: 3890–3899.
Fromigue O., Lagneaux L., Body J.J. Bisphosphonates induce breast cancer cell death. J. Bone Miner. Res. 2000, 15: 2211–2221.
Yoneda T., Michigami T., Yi B., Williams P.J., Niewolna M., Hiraga T. Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer 2000, 88: 2979–2988.
Balena R., Toolan B.C., Shea M., et al. The effects of 2-year treatment with the amino bisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strengh in ovariectomized nonhuman primates. J. Clin. Invest. 1993, 92: 2577–2586.
Storm T., Steiniche T., Thamsborg G., Melsen F. Changes in bone histomorphometry after long-term treatment with intermittent cyclic etidronate for postmenopausal osteoporosis. J. Bone Miner. Res. 1993, 8: 199–208.
Marie P.J., Lomri A., Sabbagh A., Basle M. Culture and behavior of osteoblastic cells isolated from normal trabecular bone surfaces. In Vitro Cell Dev. Biol. Anim. 1989, 25: 373–380.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxic assays. J. Immunol. Methods 1983, 65: 55–63.
West D.C., Sattar A., Kumar S. A simplified in situ solubilization procedure for determination of DNA and cell number in tissue cultured mammalian cells. Anal. Biochem. 1985, 147: 289–295.
Fromigue O., Marie P.J., Lomri A. Differential effects of transforming growth factor β2, dexamethasone and 1,25-dihydroxyvitamin D on human bone marrow stromal cells. Cytokine 1997, 9: 613–623.
Stein G.S., Lian J.B., Owen T.A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990, 4: 3111–3123.
Felix R., Fleisch H. Increase in alkaline phosphatase activity in calvaria cells cultured with diphosphonates. Biochem. J. 1979, 183: 73–81.
Vitte C., Fleisch H., Guenther H.L. Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology 1996, 137: 2324–2333.
Giuliani N., Pedrazzoni M., Negri G., Passeri G., Impicciatore M., Girasole G. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone 1998, 22: 455–461.
Tsuchimoto M., Azuma Y., Higuchi O., et al. Alendronate modulates osteogenesis of human osteoblastic cells in vitro. Jpn. J. Pharmacol. 1994, 66: 25–33.
Reinholz G.G., Getz B., Pederson L., et al. Bisphosphonates directly regulate cel proliferation, differentiation and gene expression in human osteoblasts. Cancer Res. 2000, 60: 6001–6007.
Reid I.R., Wattie D.J., Evans M.C., Gamble G.D., Stapleton J.P., Cornish J. Continuous therapy with pamidronate, a potent bisphosphonate, in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 1994, 79: 1595–1599.
Filipponi P., Cristallini S., Rizzello E., et al. Cyclical intravenous clodronate in postmenopausal osteoporosis: results of a long-term clinical trial. Bone 1996, 18: 179–184.
Tsai K.S., Hsu S.H., Yang R.S., Cheng W.C., Chieng P.U. The effectiveness of cyclic and continuous oral clodronate therapy on bone density and markers in osteopenic postmenopausal women. Calcif. Tissue Int. 1999, 64: 384–388.
Boutsen Y., Jamart J., Esselinckx W., Devogelaer J.P. Primary prevention of glucocorticoid-induced osteoporosis with intravenous pamidronate and calcium: a prospective controlled 1-year study comparing a single infusion, an infusion given once every 3 months, and calcium alone. J. Bone Miner. Res. 2001, 16: 104–112.
Adamson B.B., Gallagher S.J., Byars J., Ralston S.H., Boyle B.F. Mineralization defects with pamidronate therapy for Paget’s disease. Lancet 1993, 342: 1459–1460.
Body J.J. Current and future directions in medical therapy. Cancer 2000, 88: 3054–3058.
Sato M., Grasser W., Endo N., et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Invest. 1991, 88: 2095–2105.
Carano A., Teitelbaum S.L., Konsek J.D., Schlesinger P.H., Blair H.C. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J. Clin. Invest. 1990, 85: 456–461.
Green J.R., Müller K., Jaeggi K.A. Preclinical pharmacology of CGP42’446, a new potent, heterocyclic bisphosphonate compound. J. Bone Miner. Res. 1994, 9: 745–751.
Ralston S.H., Thiebaud D., Herrman Z., et al. Dose-response study of ibandronate in the treatment of cancer-associated hypercalcaemia. Br. J. Cancer 1997, 75: 295–300.
Body J.J., Lortholary A., Romieu G., Vigneron A.M., Ford J. A dose-finding study of zoledronate in hypercalcemic cancer patients. J. Bone Miner. Res. 1999, 14: 1557–1561.
Frith J.C., Monkkonen J., Blackburn G.M., Russell R.G., Rogers M.J. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5’-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J. Bone Miner. Res. 1997, 12: 1358–1367.
Plotkin L.I., Weinstein R.S., Parfitt A.M., Roberson P.K., Manolagas S.C., Bellido, T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 1999, 104: 1363–1374.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fromigué, O., Body, J.J. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest 25, 539–546 (2002). https://doi.org/10.1007/BF03345497
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345497