Abstract
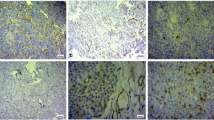
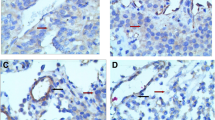
Microvessel density (MVD) represents a measure of angiogenesis and may be used as an indicator of neoplastic aggressiveness. Vascular endothelial growth factor (VEGF) plays a pivotal role as angiogenic promoter by stimulating endothelial cell proliferation and migration and enhancing vascular permeability. The aim of this study was to investigate MVD and VEGF expression in human pituitary adenomas and normal pituitary gland tissues by immunohistochemistry, and to correlate data with clinical characteristics. Fragments from 46 pituitary adenomas (18 non-functioning, 12 ACTH-secreting, 12 GH-secreting, 4 PRL-secreting) and 19 specimens of normal anterior pituitary gland obtained at surgery were evaluated. MVD in normal anterior pituitary was significantly higher than in tumors (69.2±28.5 vs 29.3±19.7; p<0.0001). Within adenomas, no difference was found in MVD when different histotype, size, sex, age, rate of recurrence or medical pre-surgical treatment were considered. The degree of vascularity was somewhat related only to clinical invasiveness, as evaluated by pre-surgical MRI grading (grade 0 p<0.05 vs grade 1 and vs grade 2). No statistically significant difference in VEGF expression was found between normal tissue and adenomas and among tumors of different histotype (p= 0.3978). Size, sex, age, rate of recurrence and medical pre-surgical treatment did not influence VEGF expression. No correlation was found between MVD and VEGF expression. In conclusion, MVD was reduced in pituitary adenomas with respect to normal gland. VEGF expression is however well preserved in adenomas and this might contribute to adequate tumoral vascular supply with complex mechanisms other than endothelial cells proliferation.
Similar content being viewed by others
References
Folkman J. What is the evidence that tumors are angiogenesis dependent? J. Nat. Cancer Inst. 1990, 82: 4–6.
Horak E.R., Leek R., Klenck N. et al. Angiogenesis, assessed by pletelet/endothelial cell adhesion molecule antibodies, as indicator of node metastasis and survival in breast cancer. Lancet 1992, 340: 1120–1124.
Macchiarini P., Fontanini G., Hardin M.J., Squartini F., Angeletti C.A. Relation of neovascularization to metastasis of non-small cell lung cancer. Lancet 1992, 340: 145–146.
Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999, 77: 527–543.
Brown L.F., Detmar M., Claffey K. et al. Vascular permeability factor/vascular endothelial growth factor: A multifunctional angiogenic cytokine. EXS 1997, 79: 233–269.
Klagsbrun M., D’Amore P.A. Vascular endothelial growth factor and its receptor. Cytokine Growth Factor Rev. 1996, 7: 259–270.
Brown L.F., Berse B., Jackman R.W. et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptor in breast cancer. Hum. Pathol. 1995, 26: 86–91.
Volm M., Koomagi R., Matter J. Prognostic value of vascular endothelial growth factor and its receptor flk-1 in squamous cell lung cancer. Int. J. Cancer 1997, 74: 64–68.
Brown L.F., Berse B., Jackman R.W. et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptor in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993, 53: 4727–4735.
Turner H.E., Nagy Z., Gatter K., Esiri M.M., Wass J., Harris A.L. Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: relationship to tumor behaviour. Br. J. Cancer 2000, 82: 1441–1445.
Turner H.E., Nagy Z., Gatter K., Esiri M.M., Harris A.L., Wass J. Angiogenesis in pituitary adenomas and the normal pituitary gland. J. Clin. Endocrinol. Metab. 2000, 85: 1159–1162.
Turner H.E., Nagy Z., Gatter K., Esiri M.M., Harris A.L., Wass J. Angiogenesis in pituitary adenomas — relationship to endocrine function, treatment and outcome. J. Endocrinol. 2000, 165: 475–481.
Vidal S., Kovacs K., Horvath E., Scheithauer B.W., Kuroki T., Lloyd R.V. Microvessel density in pituitary adenomas and carcinomas. Virchows Arch. 2001, 438: 595–602.
Lohrer P., Gloddek J, Hopfner U. et al. Vascular endothelial growth factor production and regulation in rodent and human pituitary. Neuroendocrinology 2001, 74: 95–105.
Berkman R.A., Merrill M.J., Reinhold W.C. et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J. Clin. Invest. 1993, 91: 153–159.
Nishikawa R., Cheng S.Y., Nagashima R., Huang H.J., Cavenee W.K., Matsutani M. Expression of vascular endothelial growth factor in human brain tumors. Acta Neuropathol. 1998, 96: 453–462.
Lloyd R.V., Scheithauer B.W., Kuroki T., Vidal S., Kovacs K., Stefaneau L. Vascular Endothelial Growth Factor (VEGF) expression in human pituitary adenomas and carcinomas. Endocr. Pathol. 1999, 10: 229–235.
Nakayama K., Kanzai A., Takebayashi Y. et al. Different features of angiogenesis between ovarian and breast carcinoma. Cancer Lett. 2001, 170: 161–167.
Sauter E.R., Nesbit M., Watson J.C., Klein-Szanto A., Litwin S., Herlyn M. Vascular endothelial growth factor is a marker of tumor invasion and metastasis in squamous cell carcinomas of the head and neck. Clin. Cancer Res. 1999, 5: 775–782.
Ochoa A.L., Mitchner N.A., Paynter C.D., Morris R.E., Ben-Johnathan N. Vascular endothelial growth factor rat pituitary: differential distribution and regulation by estrogen. J. Endocrinol. 2000, 165: 483–492.
Banerjee S.K., Sarkar D.K., Weston A.P., Campbell D.R. Over expression of vascular endothelial growth factor and its receptor during the development of estrogen-induced rat pituitary tumors may mediate estrogen-initiated tumor angiogenesis. Carcinogenesis 1977, 18: 1155–1161.
Gloddek J., Pagotto U., Paez Pereda M., Arzt E., Stalla G.K., Renner U. Pituitary adenylate cyclase-activating polypeptide, interleukin-6 and glucocorticoids regulate the release of vascular endothelial growth factor in pituitary folliculostellate cells. J. Endocrinol. 1999, 160: 483–490.
Ferrara N., Leung D.W., Cachianes G., Winer J., Henzel W.J. Purification and cloning of vascular endothelial growth factor secreted by pituitary folliculostellate cells. Methods Enzymol. 1991, 198: 391–405.
Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptor. FASEB J. 1999, 13: 9–22.
Carmeliet P., Moons L., Luttun A. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001, 7: 575–583.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viacava, P., Gasperi, M., Acerbi, G. et al. Microvascular density and vascular endothelial growth factor expression in normal pituitary tissue and pituitary adenomas. J Endocrinol Invest 26, 23–28 (2003). https://doi.org/10.1007/BF03345118
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345118