Abstract
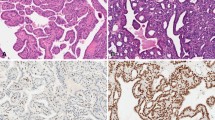
E-cadherin and catenins play a major role in neoplastic cell behavior as a suppressor of invasion and/or metastasis. The aim of this study was to determine E-cadherin, α-catenin and β-catenin expressions in papillary thyroid carcinoma (PTC) and to correlate the results of expression to initial clinicopathological parameters and clinical outcome. Forty-one cases (mean age 37.3±11.2 yr) with PTC were studied. Patients were followed-up with a mean period of 47.6±27.0 months. A retrospective immunohistochemical analysis of E-cadherin, α-catenin and β-catenin was performed on paraffin-embedded tissue sections. Tissues from ten patients with benign goiter were used as controls. E-cadherin, α- and β-catenin immunoreactivities were found in 80% (33/41), 76% (31/41) and 97% (40/41) of patients respectively. No correlation was found between E-cadherin, α- and β-catenin immunoreactivities and sex, local invasion or lymphatic spread at the time of initial examination. Distant metastases and/or local recurrences developed in 6 patients during follow-up. Recurrences/metastases developed both E-cadherin, α- and β-catenin positive and negative primary tumors. Disease-free survival curves according to Kaplan-Meier analysis and log-rank test did not show any significant differences between E-cadherin, α- and β-catenin positive and negative patients. According to our findings, E-cadherin, α- and β-catenin expressions may not add any valuable information to the follow-up in a subgroup of PTC patients with a relatively benign course.
Similar content being viewed by others
References
Pötter E., Bergwitz C., Brabant G. The cadherin-catenin system: Implications for growth and differentiation of endocrine tissues. Endocr. Rev. 1999, 20: 207–239.
Koch P.J., Franke W.W. Desmosomal cadherins: Another growing multigene family of adhesion molecules. Curr. Opin. Cell. Biol. 1994, 6: 682–687.
Schmidt A., Heid H.W., Schafer S., et al. Desmosomes and cytoskeletal architecture in epithelial differentiation: Cell type-specific plaque components and intermediate filament anchorage. Eur. J. Cell. Biol. 1994, 65: 229–245.
Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996, 84: 345–357.
Lewis J.E., Jensen P.J., Johnson K.R., Wheelock M.J. E-cadherin mediates adherens junction organisation through protein kinase C. J. Cell. Sci. 1995, 108: 3615–3621.
Gumbiner B.M. Signal transduction of beta-catenin. Curr. Opin. Cell. Biol. 1995, 7: 634–640.
Riethmacher D., Brinkmann V., Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc. Natl. Acad. Sci. USA 1995, 91: 8263–8267.
Frixen U.H., Behrens J., Sachs M., et al. E-cadherin mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell. Biol. 1991, 113: 173–185.
Jiang W.G. E-cadherin and its associated protein catenins, cancer invasion and metastasis. Br. J. Surg. 1996, 83: 437–446.
Perl A.K., Wilgenbus P., Dahl U., et al. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392: 190–193.
Bohm M., Totzeck B., Birchmeier W., Wieland I. Differences of E-cadherin expression levels and patterns in primary and metastatic human lung cancer. Clin. Exp. Metastasis 1994, 12: 55–62.
Gabbert H.E., Mueller W., Schneiders A., et al. Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int. J. Cancer 1996, 69: 184–189.
Ihara A., Koizumi H., Hashizuma R., Uchikoshi T. Expression of epithelial cadherin and α-, β-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology 1996, 23: 1441–1447.
Katagiri A., Watanebe R., Tomita Y. E-cadherin expression in renal cell cancer and its significance in metastasis and survival. Br. J. Cancer 1995, 71: 376–379.
Tamura S., Shiozaki H., Miyata M., et al. Decreased E-cadherin expression is associated with haematogenous recurrence and poor prognosis in patients with squamous cell carcinoma of the oesophagus. Br. J. Surg. 1996, 83: 1608–1614.
Risinger J.I., Berchuck A., Kohler M.F., Boyd J. Mutations of the E-cadherin gene in human gynaecologic cancers. Nat. Genet. 1994, 7: 98–102.
Brabant G., Hoang Vu C., Cetin Y., et al. E-cadherin: A differentiation marker in thyroid malignancies. Cancer Res. 1993, 53: 4887–4993.
Scheumman G.F., Hoang-Vu C., Cetin Y., et al. Clinical significance of E-cadherin as a prognostic marker in thyroid carcinomas. J. Clin. Endocrinol. Metab. 1995, 80: 2168–2172.
Yap A.S., Stevenson B.R., Keast J.R., Manley S.W. Cadherin-mediated adhesion and apical membrane assembly define distinct steps during thyroid epithelial polarization and lumen formation. Endocrinology 1995, 136: 4672–4680.
Serini G., Trusolino L., Saggiorato E., et al. Changes in integrin and E-cadherin expression in neoplastic versus normal thyroid tissue. J. Natl. Cancer Inst. 1996, 88: 442–449.
von Wasielewski R., Janssen D., Mengel M., et al. Expression of E-cadherin and β-catenin in thyroid cancer. J. Endocrinol. Invest. 1998, 21 (suppl): 17, (abstract).
Huang S.H., Wu J.C., Chang K.J., Liaw K.Y., Wang S.M. Expression of the cadherin-catenin complex in well differentiated human thyroid neoplastic tissue. Thyroid 1999, 9: 1095–1103.
von Wasielewski R., Rhein A., Werner M., et al. Immunohistochemical detection of E-cadherin in differentiated thyroid carcinomas correlates with clinical outcome. Cancer Res. 1997, 57: 2501–2507.
Wangelbach S., Sternheim E., Bittinger F., et al. Prognostic value of E-cadherin in papillary thyroid carcinoma. Chirurg 1998, 69: 186–190.
Cerrato A., Fulciniti F., Avallone A., et al. α- and β-catenin expression in thyroid carcinomas. J. Pathol. 1998, 185: 267–272.
Böhm J., Niskanen L., Kiraly K., et al. Expression and prognostic value of alpha, beta, and gamma-catenins in differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2000, 85: 4806–4811.
Hedinger C., Williams E.D., Sobin L. The WHO histological classification of thyroid tumors. Cancer 1989, 63: 908–911.
Ain K.B. Papillary thyroid carcinoma. Endocrinol. Metab. Clin. North Am. 1995, 24: 711–760.
Norton A.J., Jordan S., Yeomans P. Brief high-temperature heat denaturation (pressure cooking): A simple and effective method of antigen retrieval for routinely processed tissues. J. Pathol. 1994, 173: 371–379.
Lee Y.C., Wu C.T., Chen C.S., Chang Y.L. E-cadherin expression in surgically-resected non-small cell lung cancers-a clinicopathological study. Thorac. Cardiovasc. Surg. 2000, 48: 294–299.
Soares P., Berx G., Van Roy F., Sobrinho-Simoes M. E-cadherin gene alterations are rare events in thyroid tumors. Int. J. Cancer 1997, 70: 31–38.
Hoie J., Stenwing A.E., Kullman G., Lindegaard M. Distant metastases in papillary thyroid cancer. A review of 91 patients. Cancer 1988, 61: 1–6.
Grebe S.K.G., Hay I.D. Follicular cell-derived thyroid carcinomas. In: Arnold A (Ed.), Endocrine Neoplasms. Kluwer Academic Publishers, Boston, 1997, p. 91.
Nakanishi Y., Ochiai A., Akimoto S., et al. Expression of E-cadherin, α-catenin, β-catenin and plakoglobin in esophageal carcinomas and its prognostic significance: immunohistochemical analysis of 96 lesions. Oncology 1997, 34: 148–165.
Bongiorno P.F., Kasspooles M., Lee S.W., et al. E-cadherin expression in primary and metastatic neoplasms and in Barrett’s oesophagus. Br. J. Cancer 1995, 71: 166–172.
Nawrocki B., Polette M., Van Hengel J., et al. Cytoplasmic redistribution of E-cadherin-catenin adhesion complex is associated with down-regulated thyrosine phosphorilation of E-cadherin in human bronchopulmonary carcinomas. Am. J. Pathol. 1998, 153: 1521–1530.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kapran, Y., Özbey, N., Molvalilar, S. et al. Immunohistochemical detection of E-cadherin, α- and β-catenins in papillary thyroid carcinoma. J Endocrinol Invest 25, 578–585 (2002). https://doi.org/10.1007/BF03345079
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345079